Introduction
Natural nanoparticles (NPs) have existed in environment since the beginning of Earth's history as volcanic dust, most natural waters, soils, and sediments; they are generated by geological and biological processes, and while some can be toxic, organisms have evolved in an environment containing them.1,2 Nanotechnology represents the design, production and application of materials at atomic, molecular and macromolecular scales, in order to produce new nanosized materials. Nanomaterials are defined as materials containing man-made nanoparticles or having nanostructured surface topography. Manufactured NPs have complex colloid and aggregation chemistry, affected by particle shape, size, surface area and surface charge, as well as their adsorption properties; on the other hand, pH, ionic strength, water hardness, and organic matter alter aggregation and influence their toxicity.3-8 Most living organisms are exposed to NPs through the gastrointestinal tract, the lungs, and the skin. Once into the body, NPs interact with macromolecules of the cell organelles, mainly with proteins and nucleic acids, establishing biological interfaces that depend on colloidal forces as well as dynamic biophysicochemical interactions.9
TiO2-NPs are a fine white powder, often used as pigments or additives for ceramics, paints, paper, plastics, food, sunscreens, and toothpaste.10 Thus, organisms are exposed to TiO2-NPs and may develop toxic effects.8,11 To study their toxicity, they are administered by the oral,12 intravenous,13-15 intraperitoneal16,17 and inhalation18 routes in mice and rats. The toxicity of TiO2-NPs has mainly been studied in vitro.19-22
The main function of kidneys, in mammals, is the excretion of metabolic end products from the body, and the regulation of extracellular fluid volume and electrolyte composition.23 Their high blood flow, combined with their ability to concentrate solutes, exposes them to high concentration of xenobiotics present in the systemic circulation. Because of the rich blood supply of the kidneys, in relation to their mass, this organ is particularly liable to damage by toxic substances. Xenobiotics are physiologically concentrated in the renal tissue leading to its functional impairment.
Since the studies on renal effects of TiO2 nanoparticles (NPs) are relatively scarce, we decided to investigate the in vivo effects with the hypothesis that administration of a single dose of TiO2-NPs would produce some of the effects of these NPs, in the kidneys of adult male rats. These studies would help to reinforce assessment to the risk of exposure to living beings, since nanomaterials are found in the environment,24,25, 26, 27 in food and consumer products,28-32 at work,33-36 and even from implant release.37
Materials and methods
Animals
Male adult Wistar rats (270 ± 17g) were used and maintained in stainless steel cages with a 12 h light/dark regime. We used 6 animals per experimental group (n = 6). The rats were handled according with the Guiding Principles in the Use of Animals in Toxicology.
Chemicals
γ-L-glutamyl, L-glycylprolyl, and L-alanyl p-nitroanilides were of analytical grade and were purchased from Sigma Chemical (St. Louis, MO). Titanium dioxide nanoparticles (TiO2-NPs) of less than 100 nm were obtained from (Sigma-Aldrich). Particle size and morphology were measured using scanning electron microscopy (JEOL LV 5900), operating at 20 kV. An image of the TiO2-NPs used in this study is shown in Figure 1. A stock 2 suspension of TiO2-NPs was prepared in distilled water (30 mg/mL) and, before administration, it was diluted to desired concentration also in distilled water. All other reagents were of analytical grade.
Experimental Design
Treatment. The rats were divided in two groups: one group was treated with a single and intravenous dose of TiO2-NPs (5 mg/kg of body weight) and other group with an equivalent dose of sodium chloride (control rats). The dose used in our study (5 mg/kg of body weight) and the administration route (intravenous) are the same as used in other study in 2008,38 and is equivalent to the 8.5% of the Letal dose 50, also by the intravenous route for rats.39,40 The highest concentration of TiO2-NPs was found in liver, spleen, lung and kidney at 24 hours after intravenous injection, and returned to control values by day 14 (kidneys).38
The groups were kept in metabolic cages with food and water ad libitum, and at room temperature (24 ± 1 °C). The urine was continuously collected, in vessels attached to the metabolic cages,from0to5h,from5to24h,from24to48h,andfrom 48 to 72 h. The sampling times were selected mainly to evaluate renal biomarkers of early damage. Furthermore these sampling times are based in the high concentration of TiO2 NPs that the rats kidneys present at 24 hours post-administration i.v.38
Biochemical assays. All parameters studied were measured in the collected urine. The specific activity of γ-glutamyltranspeptidase (EC 2.3.2.2) was determined in 50 mM Tris-HCl, pH 9.0, 10 mM MgCl2, with 20 mM glycylglycine and γ-glutamyl-p nitroanilide as substrate, in a spectrophotometer at 405 nm.41 The specific activity of dipeptidylaminopeptidase-IV (EC 3.4.14.5) was assayed in 50 mM Tris-Cl, pH 8.0, with glypro-p-nitroanilide as substrate, also at 405 nm42 in a spectrophotometer. The enzymatic activities were carried out at room temperature (25 ± 1 °C). These assays were carried out in 0.5 mL final incubation volume. The initial enzymatic rates were calculated from continuous recording, in duplicate, in a UV/VIS spectrophotometer (Varian-DMS 80).
Protein was measured with the Folin phenol reagent using bovine serum albumin as standard.43 We also measured: the volume, the concentration of creatinine,44 the pH in a pH meter, the osmolatity in a microsmometer (μOsmette), the concentration of glucose in a spectrophotometer (Trinder, 1969) and the concentration of sodium with a flame photometer (Corning Flame Photometer 410).
Statistical analysis
We calculated the significance of the differences between group means with the two-tailed Student's t-test for grouped data with ANOVA pos-test of the urinary parameters, using the software Prism 4 (GraphPad Software Inc.); graphs were produced using Slide Write Plus version 4.0 for Windows (Advanced Graphics Software Inc).
Results and Discussion
In the last decade, most of the nanotoxicity studies have generally focused on culture cells and, among them, in kidney cells.45-48 However, there is a growing need for in vivo research on the effects of nanoparticles.49,50
In this study the administration of a single dose of TiO2-NPs (5 mg/kg, intravenous) altered the following parameters, as a reflection of their renal effects:
The effects of TiO2 -NPs on the enzymatic activity of γ-glutamyltranspeptidase in the urine of rats
Titanium dioxide nanoparticles increased significantly (p < 0.05) the specific activity of γ-glutamyltranspeptidase by 6.4 ± 1.0 vs 64.4 ± 10.7 (0 to 5 h); 4.3 ± 0.2 vs 63.3 ± 9.6 (5 to 24 h); 4.1 ± 0.2 vs 40.9 ± 0.6 (24 to 48 h), and 3.4 ± 0.3 vs 48.3 ± 3.4 nmol p-nitroanilide/min x mg of protein (48 to 72 h), respectively, compared with the control group, as depicted in Figure 2.

Figure 2 The effects of the intravenous administration of titanium dioxide (5 mg/kg) on the enzymatic activity of γ-glutamyltranspeptidase in the urine of rats, at different time periods, compared with control rats. The enzymatic activity is presented as nmol p-NA/min x mg of protein. The values represent the mean ± SEM, n = 6. The significance level is: ** = P<0.01; pNA: p-Nitroanilide.
The effects of TiO2 -NPs on the enzymatic activity of dipeptidylaminopeptidase-IV in the urine of rats
TiO2 nanoparticles also increased significantly the specific activity of dipeptidylaminopeptidase-IV (DAP-IV): 4.8 ± 0.6 vs 11.1 ± 0.9 from 0 to 5 h; 2.3 ± 0.2 vs 9.8 ± 0.5 from 5 to 24 h; 2.9 ± 0.2 vs 8.1 ± 0.7 from 24 to 48 h, and 3.0 ± 0.4 vs 8.5 ± 0.3 nmol p-nitroanilide/min x mg of protein from 48 to 72 h, respectively, compared with the control group (Figure 3).

Figure 3 The effects of TiO2 (5 mg/kg, intravenous) on the enzymatic activity of dipeptidylaminopeptidase-IV in the urine of rats, at different time periods, compared with control rats. The enzymatic activity is shown as nmol p-NA/min x mg of protein. The values represent the mean ± SEM, n = 6. The significance level is: ** = P<0.01; pNA: p-Nitroanilide.
We found that the earliest renal effects, on male and adult Wistar rats, were increases of the enzymatic activities of γ-glutamyltranspeptidase and dipeptidylaminopeptidase IV. These enzymes are predominantly located on the apical membrane (brush border) of proximal cells.51-54 On the other hand, enzyme activities in serum, plasma or urine are the most widely used markers of organ damage in human or experimental animals in toxicology.55-57 We believe that these effects are mainly due to a direct interaction of the TiO2-NPs with the citoplasmic membrane of the brush border cells, that line the luminal side of proximal convoluted tubules. We believe that the observed effects are mainly and initially produced by an direct interaction among TiO2 NPs-cytoplasmic membrane, of the brush border cells that line the luminal side of renal tubules, due to their small size and the lack of electric charge, making it easier to absorb and therefore, the interaction and disruption is produced on the cytoplasmic membranes. This interpretation is supported by the effects of cationic NPs and TiO2 NPs described in model membranes58-67 and in intact cells.68-73 These authors describe their findings as related to the physical disruption of model biological membranes and living cell membranes, at nanoscopic scale, by the chemical properties of nanomaterials that generate "nanoholes" in the membranes and decrease their stability. These interactions are established with the biological interfaces and depend on colloidal forces as well as dynamic biophysicochemical parameters.8,9
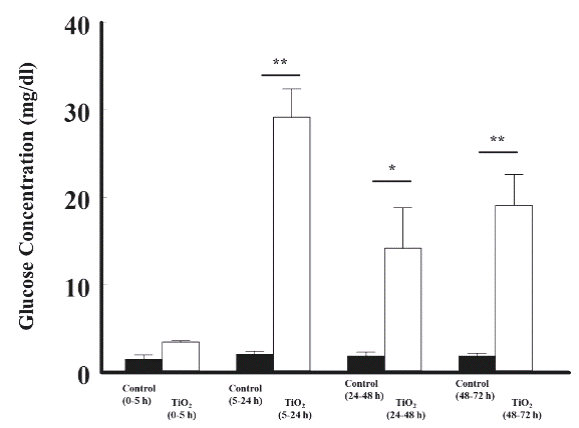
The effects of TiO2 -NPs on the concentration of glucose in the urine of rat
TiO2 nanoparticles increased significantly the glucose concentration: 29.2 ± 3.2 vs 2.1 ± 0.3 from 5 to 24 h; 14.2 ± 4.6 vs 1.9 ± 0.4 from 24 to 48 h, and 19.1 ± 3.5 vs 1.9 ± 0.3 mg/dl from 48 to 72 h, respectively, compared with the control group (Figure 4).
The effects of TiO2 -NPs on the concentration of sodium in the urine of rats
TiO2 nanoparticles increased significantly the concentration of urinary sodium by 35 ± 3 vs 85 ± 17 (5 to 24 h), 31 ± 3 vs 104 ± 9 (24 to 48 h), and 35 ± 6 vs 126 ± 8 mEq/l (48 to 72 h), respectively, compared with the control group, as shown in Figure 5.
The effects of TiO2 -NPs on urinary osmolarity of rats
Titanium dioxide nanoparticles increased significantly the osmolarity of urine by 311 ± 13 vs 580 ± 19 (5 to 24 h), by 309 ± 6 vs 434 ± 44 (24 to 48 h), and by 361 ± 14 vs 516 ± 7 mOsmol/l (48 to 72 h), respectively, compared with the control group, as shown in Figure 6.

Figure 6 The effects of TiO2 (5 mg/kg, intravenous) on urinary osmolarity of rats, at different time periods, compared with control rats. The osmolarity is shown as mOsmoles/liter. The significance level is: ** = P<0.01.
Briefly, sodium reabsorption takes place in various nephron segments. The proximal tubule is responsible for reabsorption of about 67 % of the filtered sodium load. Sodium enters the proximal tubular cell via a series of carriers that also transport other solutes.74 Thus, there are specific sodium-glucose, sodium-phosphate, and several different sodium-amino acid cotransporters. In the thick limb ascending of Loop Henle, the sodium is reabsorbed (25 %) by Na-K-2Cl co-transporter (symporter) across the membrane of the lumen. In the distal convoluted tubule sodium is transported and reabsorbed (5 %) against an electrochemical gradient by Na-Cl symporters.75
The disruption of the citoplasmic membrane by TiO2-NPs would alter the function of the cotransporters of sodium-glucose76,77 mainly the rat rSGLT278 and SGLT-5 in humans,79 as well as ion cotransporters mainly the Na-K-Cl cotransporter80-85 mainly the Na-K-Cl cotransporter.86 These transporters are the major participants in urine osmolarity.87 Those time-related effects of TiO2 NPs were detected after the enzymuria, as increased concentration of the urinary glucose (glucosuria), and increased concentration of the urinary sodium (hipernatriuria), along with increased of urine osmolality. The effects would be due to dysregulation of these nephron cotransporters
We do not rule out the participation of oxidative stress on the renal effects generated by TiO2 that have been reported.88-91 However, we believe that oxidative stress by titanium dioxide would be generated at later times, as described by Escárcega-González.92
The effects of titanium dioxide on other urinary parameters
TiO2 did not modify significantly the concentration of protein, the concentration of creatinine, the volume nor the pH of urine; likewise, titanium dioxide did not modify significantly the water and food intakes nor the body weigh, as depicted in Table 1.
Table 1 The effects of titanium dioxide (5 mg/kg body weight) on other urinary and general parameters studied.
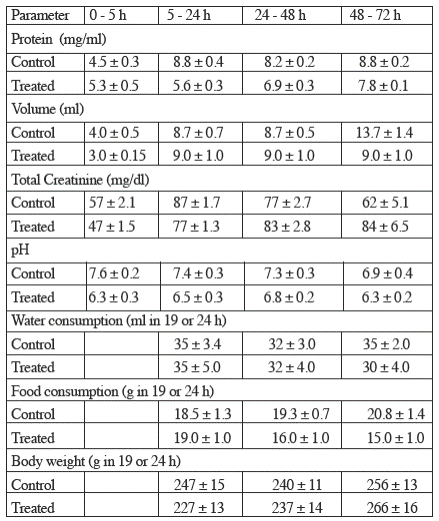
Values represent the mean ± sem (n=6).
Figure 7 summarizes the possible mechanisms of disruption in the cytoplasmic membrane of apical cells. Along the renal tubules, TiO2-NPs might change the molecular environment of 2 the two enzymes (dipeptidylaminopeptidase IV and γ-glutamiltranspeptidase) as well as of the cotransporters of Na-glucose and Na-K-Cl, and consequently, the urinary osmolality. Thus, the TiO2 NPs increase the release of the peptidases from cytoplasmic membrane reflected as their increase of activity in urine. Furthermore, the TiO2 NPs alter the function of the cotransporters and increase the urinary amount of glucose, sodium and probably other ions. This proposed mechanism, may explain the increase on the specific activity of γ-GTP y DAP-IV, similar to the studies on model membranes and nanoparticles.64,58

Figure 7 Drawing of the possible mecanisms of cytoplasmic membrane disruption by titanium dioxide nanoparticles at the luminal side of the brush border cells, all along the renal tubules of rat kidneys. γGT: γ-glutamiltranspeptidase, DAP-IV: dipeptidylaminopeptidase IV, CoT: electroneutral Na-K-Cl cotransporter, and Gluc T2: Glucose-Na cotransporter 2.
Finally, these renal effects of TiO2-NPs could be used as another biomarker of exposure to living beings, because nanomaterials are found in the environment, in food and consumer products, at work, and in humans, even from implant release.
Conclusions
The administration of a single dose (intravenous) of TiO2-NPs (5 mg/kg), to adult male rats, produced a disruption on the apical surface of the nephron cells, and allow us to dissect, timewise, initial effects (0 to 5 h) on the proximal convoluted tubule of kidneys, reflected as the urinary increase in activity of γ-glutamiltranspeptidase and dipeptidylaminopeptidase IV. These effects were followed (5 to 24 h) by the urinary increase in glucose and sodium concentration, and osmolarity. All effects remained, at least, for four days. To our knowledge we believe that this work is the first in vivo study related to early effects of TiO2 on the kidneys of rats. These renal effects could be used as another biomarker of exposure to these nanoparticles in organisms including human beings.











 nueva página del texto (beta)
nueva página del texto (beta)