Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias farmacéuticas
versión impresa ISSN 1870-0195
Rev. mex. cienc. farm vol.45 no.3 Ciudad de México jul./sep. 2014
Trabajo científico
Gelucire 39/01 as a matrix for controlled release of ranitidine hydrochloride from floating granules
Gelucire 39/01 como matriz de liberación prolongada de clorhidrato de ranitidina desde gránulos flotantes
Paulina Cruz Tamayo y Leopoldo Villafuerte Robles
Pharmacy Department, National School of Biological Sciences, National Polytechnic Institute of Mexico.
Correspondencia:
Dr. Leopoldo Villafuerte Robles
Departamento de Farmacia
Escuela Nacional de Ciencias Biológicas
Instituto Politécnico Nacional de México
Av. Wilfrido Massieu s/n esq. M. Stampa
Unidad Profesional Adolfo López Mateos
Col. Industrial Vallejo, C. P. 07738, D. F. México
E-mail: lvillaro@encb.ipn.mx; lvillarolvillaro@hotmail.com
Fecha de recepción: 28 de julio de 2014
Fecha de recepción modificaciones: 08 de diciembre de 2014
Fecha de aceptación: 13 de enero de 2015
Abstract
The application of Gelucire 39/01 for the design of floating granules of sustained-release is explored, using as a model drug ranitidine hydrochloride and different excipients to modulate the release profile. Increasing the proportion of Gelucire 39/01 decreases the release rate of the drug as well as the amount of drug released at the beginning, in an abrupt manner. The addition of different proportions of others excipients modify the release rate as well as the drug released in the initial burst. Gelucire 39/01 cannot be considered as a carrier for sustained release by itself. HPMC K15M, Tween 80, microcrystalline cellulose, Aerosil, and GalenIQ 720 allow the modulation of the release profile from the floating granules in a limited manner. All granules float individually or forming agglomerates.
Keywords: Sustained release, Dissolution profile, Granulation method, floating granules, Excipients effect, Gelucire 39/01.
Resumen
Se explora la aplicación de Gelucire 39/01 para el diseño de gránulos flotantes de liberación sostenida, utilizando clorhidrato de ranitidina como fármaco modelo y diferentes excipientes para modular el perfil de liberación. El aumento de la proporción de Gelucire 39/01 disminuye la velocidad de liberación del fármaco así como de la cantidad del fármaco liberado inicialmente, de manera abrupta. La adición de diferentes proporciones de otros excipientes modifican la velocidad de liberación así como también el fármaco liberado inicialmente, de manera abrupta. Gelucire 39/01 no puede ser considerado como un transportador para liberación sostenida por sí mismo. HPMC K15M, Tween 80, celulosa microcristalina, Aerosil y GalenIQ 720 permiten modular el perfil de liberación desde los gránulos flotantes de manera limitada. Todos los gránulos flotan individualmente o formando aglomerados.
Palabras clave: Liberación sostenida, Perfil de disolución, Método de granulación, Gránulos flotantes, efecto de excipientes, Gelucire 39/01.
Introduction
Davis has described floating drug delivery systems in 1968. These systems are used to prolong the gastric residence time. They remain buoyant in the stomach for a long time without affecting the rate of gastric emptying of other stomach contents. A floating dosage form is useful for those drugs that act locally in the proximal gastrointestinal tract (GIT). They are also useful for drugs that are unstable in lower parts of the GIT, or when they are poorly absorbed in the intestine. This type of formulation has also been used for drugs absorbed only in the initial part of the small intestine. These systems assist in the continuous release of the drug before it reaches the window of absorption, thus ensuring optimal bioavailability.1
The increased bioavailability of floating dosage forms has been attributed to the fact that the upper gastrointestinal tract is the primary site for drug absorption. Gastroretentive drug delivery systems would not be suitable for drugs, which are unstable in the stomach environment.2
The choice of one or more of the excipients for the formulation of a drug is an important step in dosage form development. An excipient may reduce manufacturing costs if it is multifunctional or can improve the patient experience of flavor masking. An excipient must be adapted to the desired dosage form, showing suitable organoleptic properties, comply with the rules of the pharmacopoeia, be easy to obtain, and work effectively. The suitable excipient will be pharmacokinetic and toxicological ideal, for pharmaceutical application. It also works well with existing equipment or with equipment, which is intended to be purchased.
Drug delivery based on lipids is a multidisciplinary approach, a congregation of organic chemistry, physics, biopharmaceutical and formulation technologies surrounding the role of fatty acids, fatty acid esters, and combinations thereof, in order to improve release and drug absorption. Lipid-based systems are used in nutritional supplements, sustained release tablets, ointments, creams and suppositories, among others.
Gelucires are vehicles derived from mixtures of mono, di- and triglycerides with polyethylene glycol (PEG) esters of fatty acids. Gelucires containing only glycerides or a mixture of glycerides and PEG esters (Gelucire 54/02, 43/01) are used in preparation of sustained release formulations. It has been reported sustained release matrices of a single unit using Gelucire 43/01, where only 1.7% of theophylline was released over a period of 20 hours. Gelucire 43/01 has also been used for the design of multiple unit floating systems of a highly water-soluble drug, diltiazem HCl. These granules were retained in the stomach for at least 6 hours; about 65% to 80% of the drug was released over 6 hours with an initial rapid release from the surface.3
The hydrophobic Gelucire 39/01 has been used as a coat to extend the release of felodipine. Caprol PEG-860 was added to this coat as a release enhancer. Caprol PGE-860, by virtue of channel formation in Gelucire coat, favored the felodipine release. The felodipine preparation encased within the Gelucire coat, was considered useful as an extended release composition for lipophilic drugs. The drug release was primarily controlled by diffusion in case of hydrophobic variants of Gelucire (Gelucire 43/01 and 54/02).4
Gelucire 43/01 beads has been used for floating delivery of metformin hydrochloride. The beads demonstrated favorable in vitro floating ability. It was found that there was no significant effect on floating ability of aged beads since they remain floating up to 8 h. Thus, it was considered that beads of Gelucire 43/01 could be used as an effective controlled delivery carrier for highly water-soluble drugs like metformin.5
A floating system for multiple units controlled release of a highly water-soluble drug, ranitidine hydrochloride, was performed using Compritol, Gelucire 50/13, and Gelucire 43/01 as lipid carriers. Ranitidine hydrochloride-lipid granules were prepared by melt granulation and were evaluated in vitro for floating and drug release behavior. These studies suggest that the hydrophobic lipid Gelucire 43/01 can be considered as an effective carrier for the design of a floating delivery system of drugs that are highly water-soluble such as ranitidine hydrochloride.6
Metronidazole-Gelucire 39/01 granules has been prepared by melt granulation technique, alone and after addition of hydroxypropylmethylcellulose K15M (HPMC K15M) or sodium cross-linked carboxymethylcellulose (Carmacel). The formulations showed that increasing proportions of Gelucire 39/01 decrease the initial fast release of the drug that stabilizes and practically ended thereafter. The granules floating times were greater than 6 hours. Gelucire 39/01, can be considered as a carrier for design of floating drug delivery systems only when mixed with dissolution enhancers that increase the permeability of the almost impermeable wax matrix.7
Ranitidine hydrochloride is a histamine H2-receptor antagonist. It is widely prescribed in active duodenal ulcers, gastric ulcers, Zollinger-Ellison syndrome, gastroesophageal reflux disease, and erosive esophagitis. Its half-life is 2.5 - 3 hours and its absolute bioavailability attains only 50%. It requires multiple doses to maintain uniform plasma levels to elicit a good therapeutic response. A sustained release formulation of ranitidine hydrochloride can provide prolonged gastric retention and increased efficacy of the dosage form.8,9,10
Floating matrix tablets of ranitidine hydrochloride have been prepared using chitosan, Carbopol and a low-density copolymer to prolong gastric residence time and increase its bioavailaility.8 Formulations of floating tablets containing the drug and HPMC K100M have been optimized using response surface methodology.9
In the same way, floating tablets have been formulated using HPMC K4M and Carbopol 934 and some other hydrophilic polymers. In this case, in combination with sodium bicarbonate and citric acid which were used as gas generating agents.10,11 Floating tablets of ranitidine hydrochloride have also been prepared employing a lipid solid dispersion, obtained by spray drying technique, with Gelucire 50/13 and Compritol 888 along with HPMC K100M and lactose. The floating approach was achieved by use of Sodium bicarbonate. Formulations containing Gelucire 50/13 and Compritol 888 alone failed to float.12
In a previous work, it was investigated the use of Gelucire 39/01 as a controlled release agent, in combination with the hydrophilic gelling polymer hydroxypropyl methylcellulose (HPMC K15M) and with sodium cross-linked carboximethylcellulose (Carmacel). The results were satisfactory only for tablets and not for the granules.7
Given the short half-life and low bioavailability of ranitidine, it is the aim of this work to explore an increase in efficacy of this drug through floating granules with prolonged drug release. Gelucire 39/01 added of some other excipients acting as release modifiers would provide the floating properties and the modulation of drug release. Two granulation methods are investigated, melting Gelucire to agglomerate the powder (melt granulation) and agglomeration through mortar blending-Kneading of Gelucire and the drug (dry granulation).
Materials and methods
The drug ranitidine hydrochloride batch 201 0012 was obtained from DVA Mexicana. The excipient Gelucire® 39/01 (waxy solid, melting point=39°C, HLB=01), batch 5E0907-2 and GE2007-2 was obtained from Lubrizol Mexico. Tween 80, batch 00115201, was obtained from Globe Chemicals. Methocel K15M CR premium USP (hydroxypropyl methylcellulose - HPMC K15M), batch MC28912NO1 and Methocel K4M were obtained from Dow Chemical. Agglomerated spherical isomalt, disaccharide alcohol in a 1:1 ratio of 6-O-α-D-glucopyranosyl-D-sorbitol and 1-O-α-D-glucopyranosyl-D-mannitol dihydrate, GalenIQ 720, batch 2548709 was obtained from Beneo Palatinit. Microcrystalline cellulose type 102, batch DOU-458 was obtained from FMC Mexicana. Amorphous anhydrous colloidal silicon dioxide, Aerosil 200, was obtained from Evonik Degussa Mexico.All of them were used as received.
Preparation of floating granules and tablets
Floating granules containing ranitidine hydrochloride were prepared using the melt granulation technique. The drug:lipid ratios used to prepare the different formulations were 1:1, 1:2, 1:3, 1:4 and 1:5. To study the effect of HPMC K15M it was added at ratios of 3%, 5%, 7%, 10% and 19%. Tween 80 was used at ratios of 1%, 3% and 5%. To study the influence of other excipients it was used the dry granulation method in a mortar. The studied excipients were microcrystalline cellulose, Aerosil and GalenIQ 720. Microcrystalline cellulose was used at proportions, referred to the drug, of 1:1, 1:1.5, 1:2, and 1:3. A new series of similar formulations with microcrystalline cellulose was added of 2% Aerosil. GalenIQ 720 was used at proportions, referred to the drug, of 1:1, 1:2, 1:3, 1:4 and 1:5. For the melt granulation, the lipid was melted at 50°C, and the drug or drug and additives mixture was added, mixed well, and cooled to room temperature. The mass was passed through a number 20 sieve to obtain uniform-sized granules. The cooled granules were sieved using a mesh number 16. The dry granulation process included 15 min mixing in a mortar of the drug and other excipients, a separate milling of Gelucire 39/01 and the mixing-kneading of all components in the mortar for 30 min. The obtained mass was passed through sievenumber 20.
As a reference, tablets containing 20% or 30% HPMC K4M were obtained after mixing of components for 30 min in a V-blender, compacting at a minimal compaction pressure using flat faced punch and die with diameter of 12.7 mm. 1% magnesium stearate was used as a lubricant.
In vitro evaluation of floating ability
The floating times were measured by visual observation of three repetitions.
In vitro drug release
In vitro drug release studies were carried out in triplicate using USP 36 type II dissolution test apparatus (SRII 6, Hanson Research Corporation, Chatsworth, California, USA), commonly known as the paddle method. A tablet or the equivalent quantity of granules containing 300 mg ranitidine hydrochloride was added to 900 ml HCl 0.1 N. The solubility of ranitidine.HCl in water is 660 mg/ml, because of that the total drug dissolved is considered to occur under sink conditions.13Dissolutionmedia was thermostated at 37±0.5°C and stirred at 50 rpm. Aliquots were collected periodically and replaced with fresh and prewarmed dissolution medium. The aliquots, after filtration, were analyzed using spectrophotometer at 314 nm for ranitidine hydrochloride content. Dissolution was determined in the interval of 6 hours.
Results and discussion
Ranitidine hydrochloride:Gelucire 39/01 granules
Lipidic materials like Gelucire 39/01 are considered as an alternative to polymers used in sustained release formulations because some advantages such as low melt viscosity, absence of toxic impurities, potential biocompatibility and biodegradability and prevention of gastric irritation by forming a coat around the gastric irritant.14
Together with some other, the objective of a dosage form development is to ensure the delivery of specific and reproducible amounts of pharmacologically active compounds to the body. Particularly by sustained release products, one of the goals is to maintain a steady state level of drug concentration in the blood. In doing so a balance can be achieved between the amount absorbed and that being excreted. This is attained by releasing constant quantities of the drug per unit of time, equivalent to the drug quantities being eliminated from the body; in theoretical zero order release kinetics.
Ranitidine hydrochloride:Gelucire 39/01 granules dissolving in 0.1 N HCl showed that drug release was retarded notably with an increasing amount of Gelucire 39/01 (Figure 1). As can be seen, the release profiles do not show a zero order release. All five drug:lipid ratios showed an important burst effect in the initial stage of drug release and, with exception of the lower proportion of Gelucire 39/01, a particularly slow drug release thereafter. These data would make difficult the use of Gelucire 39/01, by itself, as a sustained release excipient.
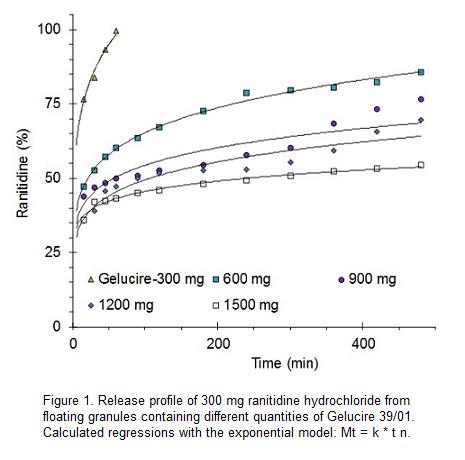
The release profiles are similar to those found in literature for metformin hydrochloride formulated with Gelucire 43/01, at proportions ranging from 1:5 to 1:15. The 4 mm metformin beads displayed a burst effect ranging from about 20% to 35%, followed by a slow release ending with drug-dissolved proportions ranging from about 30% to 60%. In spite of this, the authors concluded that beads of Gelucire 43/01 could be useful as an effective carrier for the controlled delivery, particularly for highly water-soluble drugs like metformin hydrochloride.5
This type of release profiles has been attributed to the high hydrophobicity of Gelucire. The release medium is not able to diffuse through the matrix and progress in the dosage form. Dissolution of the drug particles on the surface of the matrix allows the formation of channels, from which the drug is slowly released.
Regression parameters of release profiles of ranitidine hydrochloride are summarized in Table 1. The regressions parameters were calculated according to the exponential model. The formulations correspond to granules containing different proportions of Gelucire 39/01.
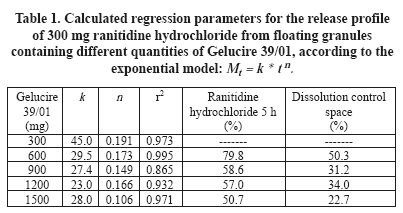
The semi-empirical exponential model describes the relationship between the fractions or percentage drug released Mt and the elapsed time t, k is the release constant or antilogarithm of the intercept in a logarithmic relationship and n the slope of the curve in a logarithmic relationship. In the case of tablets, then value is commonly used to characterize the release mechanism as Fickian diffusion, non-Fickian transport or anomalous, case II or relaxational transport and super case II or erosion related transport. This model is used to characterize drug dissolution and is normally used to investigate a proper "fit" for the experimental measurements of drug release.15
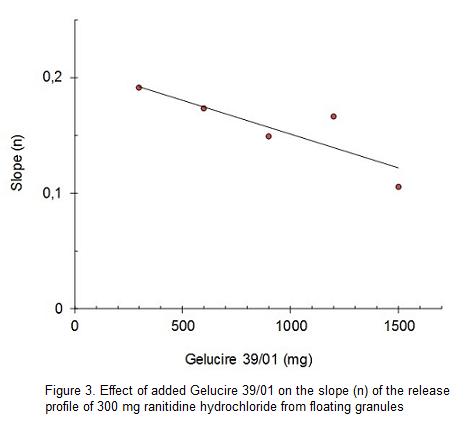
Figure 2 displays the relationship between the ranitidine hydrochloride dissolved after 5 h and the release constant (k), of ranitidine hydrochloride floating granules containing different proportions of Gelucire 39/01. Values of k can be considered as the proportion of the drug that is released without control of the release modifier or burst effect. The space between these curves can be defined as the control space or space of drug release controlled by the release modifiers. This concept is defined to discriminate the proportion of the drug released that is controlled by each formulation. This space decreases as the Gelucire 39/01 proportion increases. The drug release controlled by Gelucire 39/01 begins with 50%, at low content of Gelucire 39/01, ending with 23% at higher Gelucire 39/01 proportions. The better dissolution control space can be situated at Gelucire 39/01 values under 600 mg, for a ranitidine hydrochloride dose of 300 mg. However, in this experimental space the burst effect seems to be too high.
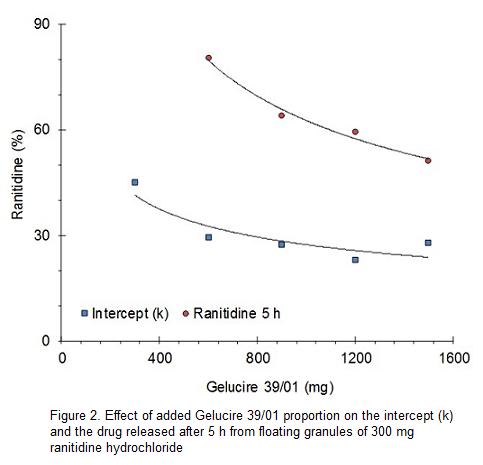
Drug release data treated according to the exponential model or Korsmeyer-Peppas equation display slopes ranging from 0.106 to 0.191. This behavior is referred by tablets as Fickian diffusion (n>0.43). If 0.43<n<0.85 the transport is not Fickian or anomalous and if n>0.85 the zero order release mechanism dominates.5
Figure 3 depicts a lineally decreasing exponent n as the Gelucire 39/01 proportion increases. Values of n or slopes of the exponential model, in a logarithmic relationship, are indicative of drug release mechanism and in a certain manner of the release rate from the granules of Gelucire 39/01. In this sense, increasing amounts of Gelucire 39/01 decrease progressively the drug release rate.
Influence of HPMC K15M on the release profile
Gelucire 39/01 can be used as an extended delivery excipient; however, it is necessary to add a release-modifying agent to improve the drug release kinetics. It means the reduction of the burst effect and the increase of the subsequent release rate. HPMC is added with the expectation to reduce the initial burst effect. The addition of HPMC K15M to granules of ranitidine hydrochloride:Gelucire 39/01 (1:3), as a release enhancer, showed an increase in drug release compared to plain drug:lipid granules, mainly expressed as a burst effect (Figure 4).

Although HPMC is used commonly as a controlled release carrier, it can also function as a swelling or disintegrating excipient. In this case, using a ranitidine hydrochloride:Gelucire 39/01 proportion of (1:3), the combination of HPMC and Gelucire 39/01 does not give the impression to display a shared effect. It seems that swelling of HPMC only breaks off the continuity of the hydrophobic domain of Gelucire 39/01, leading to faster drug release from the granules.
The addition of HPMC (Methocel K15M) to granules of metronidazole:Gelucire 39/01, as a release enhancer, showed an increase in drug release compared to plain drug:lipid granules
Similar results were observed by metronidazole:Gelucire 39/01 (1:0.8) floating granules.7 Addition of HPMC K15M as a release enhancer showed release profiles displaying values of drug dissolved after 3 hours ranging from 133 mg (26.6%) to 497 mg (99.4%). Currently, release profiles of ranitidine hydrochloride display a range of drug dissolved from 56% to 100%. The wider span of the range of metronidazole dissolved after 3 hours is attributed to a lower proportion of Gelucire 39/01 in metronidazole granules. The lower proportion of Gelucire 39/01 allowed a better deployment of the HPMC K15M effect.
HPMC K15M increases not only the drug release with time but also the burst effect. Apparently, HPMC K15M swells and allows the formation of channels from which the drug is released. All the formulations floated throughout the dissolution study in the same way as those containing only the drug and Gelucire 39/01.
Regression parameters of release profiles of ranitidine hydrochloride, according to the exponential model, are summarized in Table 2. The granules contain 300 mg ranitidine hydrochloride with 900 mg Gelucire 39/01 and different proportions of HPMC K15M.

The HPMC K15M opening of channels in the granule structure does not allow the increase of the dissolution control space of Gelucire 39/01. It shows an average of 36.7%, without any specific trend as the HPMC K15M proportion increases. The average of dissolution control space of formulations without HPMC K15M is 34.5%. The release profiles only move to somethinggreater drug release proportions.
The slope of the release profiles also display no particular trend with increasing HPMC K15M proportions. However, its average decreases from 0.157 to 0.094 after addition of HPMC K15M.
The more important change occurred after addition of HPMC K15M is the increased values of k. The average of the percentage of drug dissolved without control of the release modifier (k) increased from 30.6% to 52.1%. It is observed a clear trend to increasing values of k as the proportion of the release enhancer, HPMC K15M, increases. The addition of increasing proportions of HPMC K15M in a range of 3-19% facilitate in an important manner the initial drug dissolution with slower release rates thereafter.
Influence of Tween 80 on the release profile
Figure 5 shows the release profile of floating granules of ranitidine hydrochloride:Gelucire 39/01 (1:3), containing different proportions of Tween 80. This figure depicts an important increase in drug dissolution after addition of Tween 80. The slow drug release observed by formulations containing only Gelucire 39/01 is attributed to its hydrophobic nature (HLB 1). It might have reduced the wetting of the drug and thus, its dissolution. Addition of surfactants such as Tween 80 (HLB 15) has the intention to open some channels through the Gelucire 39/01 barrier, improving the drug release process at the solid/liquid interface.

Tween 80, a high HLB excipient, may act as a dissolution enhancer. The hypothesis is that Gelucires with low HLB can be employed to decrease the dissolution rate of drugs whileexcipients with high HLB can be used to increase it. 16
Although the aim to add Tween 80 was the increase of dissolution rate, the proportions used here (1-5%) are too high. Apparently, the proportions of Tween 80 are capable to dissolve the barrier of Gelucire 39/01, allowing a free dissolution of the drug.
The above-mentioned formulations of ranitidine hydrochloride produced floating granules with some degree of agglomeration upon contact with the dissolution medium. The presence of Tween 80 providedfloating granuleswithwater dispersion capabilities, avoiding agglomeration. These granules floated in water without the agglomeration observed before.
Influence of the preparation method
The melt granulation technique is a size enlargement process. The addition of a binder that melts or softens at relatively low temperatures is used to achieve agglomeration of solid particles in the formulation. This process can be used for preparation of sustained released dosage forms by using lipophilic polymers.17
However, the melt granulation technique may alter the aggregation state of lipid materials; it may allow the presence of metastable structures that can change with time. Solid Gelucire exists in three crystalline forms, melted Gelucire on cooling forms unstable form II which is converted into metastable form I which is finally converted into stable form I'.18
The consequences of melt agglomeration and formation of metastable structures has been observed by Gelucire 43/01. Complete melting of the Gelucire 43/01 occurs at 47°C after aging, instead of 43ºC. The energy required for melting also increased with aging. All this might be attributed to phase transformation due to crystallization of glycerides.5
In the same way, it has been observed by Gelucire 39/01 that ageing was responsible for an increase in drug release. It was observed that the release-retarding activity of Gelucire 39/01 is reduced during ageing. This was attributed to conversion of metastable form I to stable form I'.18
As an alternative to melt granulation, it was here used the agglomeration with mortar and pestle at room temperature. Figure 6 depicts the effect of preparation method on the release profile of 300 mg ranitidine hydrochloride added of 1500 mg Gelucire 39/01. Compared to melt granulation, the use of granulation with mortar and pestle reduced drug release after 5 h in about 21%. The shift to lower proportions of drug release is accompanied with an increase of the exponent (n) from 0.104 to 0.173. In spite of the important change in the drug released after 5 h, the dissolution control space does not change in an important manner. The difference between k values and the drug dissolved after 5 h is about 24% for melt granulation while it is 20% for dry granulation.
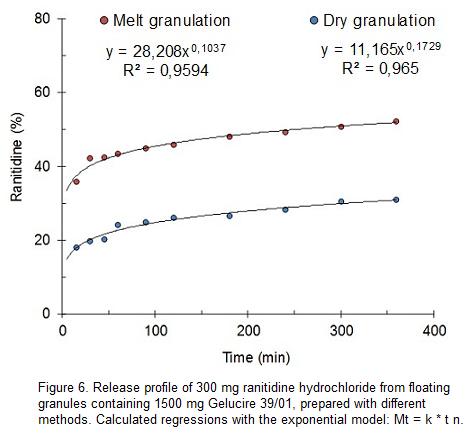
It is assumed that the metastable forms of Gelucire 39/01 obtained by melt granulation are responsible of the faster drug release. The slower release rate observed by use of mortar and pestle is attributed to a more stable or less disturbed aggregation form of the excipient.
Influence of addition of microcrystalline cellulose
The use of agglomeration in a mortar and a ranitidine hydrochloride:Gelucire 39/01 proportion of 1:5 was employed to study the effect of microcrystalline cellulose on the release profile of the drug (Figure 7). This proportion of the drug against Gelucire was selected with the expectation of higher dissolution rates after addition of the new excipients to be tested.In the same way as by addition of HPMC K15M, increasing proportions of cellulose increase the intercept of release profiles as well as the amount of ranitidine hydrochloride released after 5 h dissolution (Table 3).

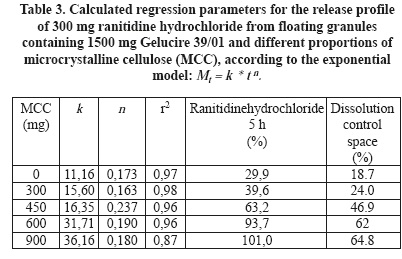
In the case of cellulose, the dissolution control space increases progressively with increasing proportions of this excipient. The dissolution control space attains greater values than formulations containing HPMC K15M. It goes from approximately 19% to 65% while in granules with HPMC K15M it displays an almost constant average of approximately 37%.
Although it was not apparent an effect of release retardation of HPMC K15M on the release profile of ranitidine hydrochloride:Gelucire 39/01 (1:3) granules, it becomes evident when compared with granules containing cellulose. The increasing amounts of HPMC K15 are responsible of an increasing initial drug release, represented by k values. However, its release retarding effect may be responsible of maintaining an almost constant dissolution control space. After addition of cellulose to ranitidine hydrochloride:Gelucire 39/01 (1:5) granules, the initial drug release increases progressively as well as the dissolution control space. Cellulose displays a similar effect on the initial drug release as HPMC K15M but without the release retarding effect. It allows the concomitant increase of the drug dissolved after 5 h and with this, the increase of the dissolution control space.
The slope of release profiles of ranitidine hydrochloride:Gelucire 39/01 (1:5) floating granules does not display any specific trend. However, it is about the double of that obtained with floating granules containing HPMC K15M.As observed before, it can also be attributed to the granulation method. This occurs in spite of a higher proportion of Gelucire 39/01 in the granules, compared to formulations containing HPMC K15M.
Influence of addition of microcrystalline cellulose combined with Aerosil
Aerosil is one of the commercially available products of colloidal silicon dioxide (SiO2). Aerosil is characterized by a relatively large specific surface area, typically 200 square meters per gram of material. The siloxane and silanol groups situated on the surface of Aerosil particles are responsible for its hydrophilic behavior.
Aerosil has been employed as moisture adsorbent, free-flow agent and glidant at a concentration of 2.0% w/w and as disintegrant influencing the water uptake. Aerosil has also been used to enhance drug dissolution. Theophylline-loaded microparticles of a lipid carrier, Precirol ATO 5, showed increasing drug release as the Aerosil concentration increased.19
Currently, Aerosil has been added to ranitidine formulations to enhance the drug dissolution from floating granules containing Gelucire 39/01 and microcrystalline cellulose. The formulations are similar to those containing microcrystalline cellulose as adjuvant but added of 2% Aerosil.
Table 4 summarizes the regression parameters of the release profiles of ranitidine hydrochloride:Gelucire 39/01 (1:5) floating granules added of different proportions of microcrystalline cellulose plus 2% Aerosil. Compared to formulations containing only MCC, the addition of 2% Aerosil increases in an important manner the burst effect or k values, from an average of 24.9% to an average of 47.9%, while decreases the values of the slopes of the curves (n) from 0.189 to 0.113. As a result, the average of the dissolution control space diminishes from 49.4% to 40.3%. This is attributed to an increased hydrophilicity of the floating granules after addition of Aerosil. Aerosil increases the availability of water inside the granules, promoting the drug dissolution. The increase ofranitidine dissolution leaves less drug to be dissolved subsequently, reducing the release rate.

Figure 8 depicts the comparative dissolution enhancing effect of Aerosil. All formulations of floating granules containing Aerosil display higher proportions of ranitidine hydrochloride dissolved after 2 h. However, the effect of Aerosil is lesser as the proportion of MCC in the formulation increases. The reduction of the dissolution enhancing effect of Aerosil can be referred to the increased hydrophilicity of the formulations due to MCC. MCC confers also hydrophilic properties to floating granules, making less noticeable the additive effect of Aerosil.

A similar effect has been observed by theophylline-loaded microparticles of a lipid carrier, Precirol ATO 5, where Aerosil 200 was added to modify the release behavior. The release enhancing effect of Aerosil was evident, the release of theophylline increased as the Aerosil 200 concentration increased.19
In the same way, Aerosil (1-5%) has been observed to improve dissolution of drugs like ibuprofen due to increased wettability. The presence of silicon dioxide has been considered to increase hydrophilicity of the drug particle, facilitating access of water during dissolution.20
Aerosil has also been used to enhance dissolution of itraconazole solid dispersions. Although no clear trend could be observed between drug loading and dissolution performance of the solid dispersions, dissolution of the powders was enhanced compared to crystalline itraconazole.21
It seems that choosing the suitable amount of Aerosil, it could be a useful excipient to modulate the release of drugs from waxy material such as Gelucire 39/01. The intention of adding Aerosil is the modulation of the release retarding effect of Gelucire 39/01 on ranitidine hydrochloride dissolution. However, Aerosil does not reduce the burst effect but increases it from k=24.9 to k=47.9, reducing at the same time the space of the drug dissolution being controlled by Gelucire 39/01. Although the Aerosil dissolution enhancing effect is clear, it is not suitable to modulate the release retarding effect of Gelucire 39/01 when accompanied of another hydrophilic excipient such as microcrystalline cellulose.
Influence of addition of GalenIQ 720
GalenIQ 720 is an agglomerated spherical isomalt for direct compression applications and capsule fillings. Its solubility in water is 25 g/100g. GalenIQ direct compressible grade 720 is recommended for all types of solid formulations. This excipient was selected as release modifier because their flow properties and passive disintegration properties. It is very low hygroscopic and heat stable; melting range: 145 to 150°C, no reaction with amino groups and no incompatibilities with API„schallenged.
The aim of addition GalenIQ 720 to Gelucire 39/01 is the formation of channels after its dissolution. GalenIQ 720 was selected because it is less soluble than the type 721. The purpose is the retardation of the initial drug release and a subsequent increase of the same, after dissolution of this release modifying excipient.
Table 5 summarizes the regression parameters for the release profiles of ranitidine hydrochloride from Gelucire 39/01 floating granules containing different proportions of GalenIQ 720. From data of k values, it is clear an important reduction of the burst effect. The values of k show an average of 9.5%, the smallest of all studied formulations. These values can be considered as acceptable. At the same time, the slope of the release profiles or n values are the greatest of all studied formulations. Both parameters complying with the original objectives. However, it could be desirable a better control of the subsequent release rate to maintain an increased drug release up to or near to 100%.

The above-mentioned results can be contrasted with those obtained with a polymeric matrix, to make evident the need for a better control of the release retarding properties of Gelucire 39/01. The selected reference is HPMC matrix tablets (100 mg) containing pelanserin hydrochloride and different proportions of Pharmatose DCL21, an excipient with similar properties as GalenIQ 720. The release profiles from these matrix tablets displayed dissolution control spaces beginning practically at 0% and dissolving after 8 h about 55-75% of the drug. Further, the slopes of release profiles displayed values varying from 0.587 to 0.95, allowing the release profiles the inference of a continuous drug release up to the total content.22
Figure 9 depicts the effect of GalenIQ 720 on ranitidine hydrochloride released after 5 h, compared to the burst effect represented by the values of k. Both parameters increase linearly as the content of GalenIQ 720 increases. As can be seen, the dissolution control space increases also progressively as the GalenIQ 720 proportion increases. The release behavior can be controlled as a combination of release rate and dissolution control space. The desired release rate can be adjusted with addition of a given proportion of GalenIQ 720, if the resulting dissolution control space is acceptable.

Tablets of ranitidine hydrochloride with HPMC K4M
As a reference to compare the controlled release properties of Gelucire 39/01, ranitidine hydrochloride tablets were prepared containing 20% and 30% HPMC K4M. Tablets containing 20% polymer were obtained at different compaction pressures just to confirm that this parameter does not affect the release behavior of HPMC K4M tablets in an important manner (Table 6). Tablets containing different proportions of HPMC K4M were prepared at 100 kg/cm2.
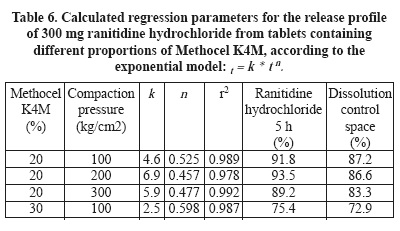
Ranitidine hydrochloride tablets compacted at 100 kg/cm2 display values of dissolution control space greater than 70%. The smaller values of k and the higher values of the slopes (n) of release profiles allow the greater dissolution control space.
Figure 10 depicts the release profiles obtained from HPMC K4M matrix tablets. The space covered by the release profiles of tablets containing 300 mg ranitidine hydrochloride and 30% polymer begins at k=2.5%, attaining 75% drug dissolution after 5 h. This release profile and its slope allow the inference that the drug release will continue in the same trend up to the total drug dissolution. These results call attention to the need for a better modulation of Gelucire 39/01, to make it a competitive useful controlled release agent.

Conclusion
Gelucire 39/01 cannot be used as itself as an extended release agent. The use of Gelucire 39/01 produces formulations with an important burst effect and a subsequent quite small drug release rate. Addition of release enhancers such as HPMC K15M, microcrystalline cellulose, Aerosil, Tween 80 and GalenIQ 720 to Gelucire 39/01 floating granules allow the modulation of drug release rates and the burst effect, however, in a limited manner. Gelucire 39/01 is suitable to maintain floating in water all the studied granules, sometimes forming agglomerates but floating freely when the formulation included Tween 80 or Aerosil. Although the here studied excipients to enhance the release profiles obtained with Gelucire 39/01 gave moderate results, the successful development of some products cited in literature stimulate to continue the search of better formulations for the controlled release from gastric retentive dosage forms based on hydrophobic Gelucires.
References
1. Jiménez I, Quirino T, Villafuerte L. Sustained delivery of captopril from floating matrix tablets. Int J Pharm. 2008; 362 (1-2): 37-43. [ Links ]
2. Jiménez-Martínez I, Domínguez-Ramírez AM, Villafuerte-Robles L. Effect of antioxidants on captopril floating matrices. Pharm Dev Tech. 2010; 15 (3): 230-40. [ Links ]
3. Shimpi S, Chauhan B, Mahadik K R, Paradkar K. Preparation and Evaluation of Diltiazem Hydrochloride-Gelucire 43/01 Floating Granules Prepared by Melt Granulation. AAPS PharmSciTech. 2004; 5 (3): 51-6. [ Links ]
4. Patil PR, Biradar SV, Paradkar AR. Extended Release Felodipine Self-Nanoemulsifying System. AAPS PharmSciTech. 2009; 10 (2): 515-23. [ Links ]
5. Jain SK, Gupta A. Development of Gelucire 43/01 Beads of Metformin Hydrochloride for Floating Delivery. AAPS PharmSciTech. 2009; 10 (4): 1128-36. [ Links ]
6. Patel D M, Patel N M, Patel V F, Bhatt D A. Floating Granules of Ranitidine Hydrochloride-Gelucire 43/01: Formulation Optimization Using Factorial Design. AAPS PharmSciTech. 2009; 8(2): E25-31. [ Links ]
7. Juárez-Soberanez D, Villafuerte-Robles L. Gelucire 39/01 as excipient for gastroretentive metronidazole sustained delivery. Int J Pharm Pharmac Scis. 2011. 3, suppl.2, 86-91. [ Links ]
8. Patel R, Singh RP, PanchalK M, Gupta S and Kumar L. Formulation and optimization of floating matrix tablet of ranitidine hydrochloride. Pharmacie Globale (IJCP). 2011; 5 (08): 1-5. [ Links ]
9. Sahoo BK, Mishra AM, Pal TK. Optimization and Validation of Modulated Release Formulation of Ranitidine HCl by Response Surface Methodology. Int J Pharm Scis Drug Res. 2011; 3(1): 13-18. [ Links ]
10. Trivedi ND, Trivedi UN, Patel MM, Patel JK, Bhandari A. Preparation and evaluation of floating matrix tablet of ranitidine. Am J drug discdev. 2011; 1 (1): 8-23. [ Links ]
11. Ravala JA, Patela JK, Lib N, Patel MM. Ranitidine hydrochloride floating matrix tablets based on low density powder: effects of formulation and processing parameters on drug release. Asian J Pharm Scis. 2007; 2 (4): 130-142. [ Links ]
12. Ashokkumar D, Mohanta GP. Formulation and in vitro evaluation of ranitidine HCl floating tablets by lipid solid dispersion spray drying technique. Int J Res Pharm Biomed Sci. 2012; 3(4): 1745-1749. [ Links ]
13. Fuertes I, Caraballo I, Miranda A, Millán M. 2010. Study of critical points of drugs with different solubilities in hydrophilic matrices. Int J Pharm.2010; 383: 138-146. [ Links ]
14. Jadhav N, Gubbi S, Kadam H Gelucires: Pharmaceutical applications. Latest Reviews. 2008; 6 (4). Available at: http://www.pharmainfo.net/reviews/gelucirespharmaceutical-applications. Accessed on June 24, 2010. [ Links ]
15. Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001; 13 (2):123-133. [ Links ]
16. Siripuram PK, Bandari S, Jukanti R, and Veerareddy P R. Formulation and Characterization of Floating Gelucire Matrices of Metoprolol Succinate. Dissol Technol. 2010; August: 34-39. Available at: http://www.dissolutiontech.com/DTresour/201008Articles/DT201008_A05.pdf. Access on July 04, 2014. [ Links ]
17. Srikanth S, Krishna KVM, Babu G. Melt granulation: an alternative to traditional granulation techniques. World J Pharm Pharm Scis. 2013; 2(5):2420-2429. [ Links ]
18. Chauhan B, Shimpi S, Mahadik KR, Paradkar A. Preparation and evaluation of floating risedronate sodium Gelucire 39/01 matrices. Acta Pharm. 2004; 54:205-214. [ Links ]
19. Albertini B, Passerini N, González-Rodríguez M L, Perissutti B, Rodriguez L. Effect of Aerosil on the properties of lipid controlled release microparticles. J Control Rel. 2004; 100: 233 - 246. [ Links ]
20. Mallick S, Pradhan SK, Chandran M, Acharya M, Digdarsini T, Mohapatra R. Study of particle rearrangement, compression behavior and dissolution properties after melt dispersion of ibuprofen, Avicel and Aerosil. Results in Pharma Sciences. 2011; 1:1-10. [ Links ]
21. Van Eerdenbrugh B, Van Speybroeck M, Molsa R, Houthoofd K, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, Van den Mooter G. Itraconazole/TPGS/Aerosil200 solid dispersions Characterization, physical stability and in vivo performance. Eur J Pharm Scis. 2009;38:270-278. [ Links ]
22. Espinosa-Ramos R, Villafuerte-Robles L. Influence of admixed lactose on pelanserin hydrochloride release from hydroxypropyl methylcellulose matrix tablets. Pharm Acta Helv. 1999; 74:65-71. [ Links ]














