1. Introduction
Today, copper is a strategic material given its great importance for the manufacture and use of electrical and electronic components (Dillon et al., 2015). Due to this, it has become essential to recover this metal from electrical circuit waste, electronic waste, vehicle parts, among others (Wang et al., 2018). The management of electronic scrap as a raw material for the recovery and production of metals and alloys, mainly copper, has become a crucial stage at an industrial level, due to the increase in electronic waste around the world (Barragan et al., 2020; Wang et al., 2017).
The problems in recycling waste electrical and electronic equipment (WEEE) have become a global concern, where it is estimated that between 20 and 25 million tons of WEEE are produced per year. For the recycling of WEEE and the recovery of metals of interest, routes have been addressed through acid leaching processes, as well as open incineration pyrometallurgical processes. However, the latter has generated serious environmental and health problems (Wang et al., 2016).
The management of pyrolysis plants helps to reduce the excessive accumulation of solid waste, as well as environmental pollution (Yazdani et al., 2019). These systems have been implemented mainly for the conversion of tires into fuel, where the metallic material can also be recovered, giving use to the secondary waste produced during the process (Labakali & Jeguirim, 2017).
In conventional pyrometallurgical melting processes, WEEE is added directly in furnaces, which produces a high degree of pollutants for the environment. An alternative to solve this problem is to use combustion processes in a closed circuit, reducing the volume of the polluting material between 80-90%, which has promoted the development and construction of incinerators or pyrolizers (Onwughara et al., 2010; Sepúlveda et al., 2010).
However, the application of pyrolizers in the treatment of WEEE produces ashes that manage to drag part of the metallic material present in the sample. An alternative to recover the metallic material deposited in these ashes is the use of gravimetric separation methods, which allow the separation of materials with different densities (Ambrós et al., 2019; Mouedhen et al., 2018).
The main operating parameters of these processes (mechanical screens, JIG screens, and Wilfley tables) take into account the physical properties of the particle, the length, and frequency of the blows, the inclination of the platform, and the water flow (Ambrós et al., 2019; Fitzpatrick et al., 2016; Wills, 2016).
Taking into account the above, in this work three gravimetric separation methods were used for the recovery of copper deposited in the ashes produced during a WEEE pyrolysis process. The results obtained in each of the cases were analyzed to determine the efficiency of each method used.
2. Materials and methods
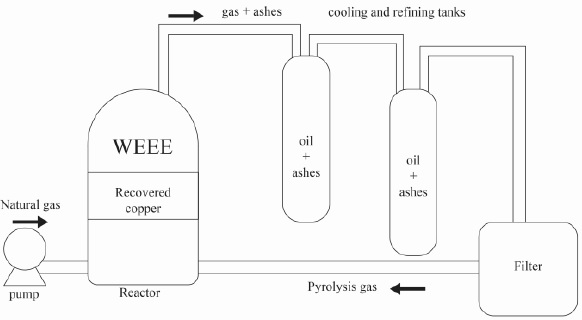
The raw material used in this work was an electrolytical copper wire covered with a vinyl polyethylene insulation ([C2H4]n), conventionally used in electrical installations in the manufacture of electrical circuits. The copper wire was subjected to a pyrolysis process inside a 250 kg capacity pyrolysis reactor (Figure 1), belonging to the Habitat A Vibe Company SAS in Bogotá, Colombia.
The process consisted of adding a load of 230 kg of copper wire and 20 kg of limestone at 900°C for 4 hours at atmospheric pressure (Lai & Lu, 2003). The samples were cooled inside the reactor. The ashes generated during the pyrolysis process were collected and taken to a homogenization stage followed by process sampling by the ASTM C702/C702M, ASTM E11-09, and ASTM C136/C136M standards, until obtaining samples of 3 kg (ASTM, 2003, 2010; Statements & Ag-, 2014).
The homogenized sample was initially analyzed using the X-ray diffraction technique (XRD) to identify the compounds present in the sample. The process was performed using a PanAlytical diffractometer with a source of Co, λ = 1.75Å, a variable angle detector, pixel with Bragg Brentano configuration, operating at 40kV and 30mA, a diffraction angre 2θ range from 1.5° to 70° and a step of 0.04 ° s-1.
The percentage by weight of metallic content in the ash samples was determined by physicochemical separation according to the following procedure:
1) 1kg of the previously homogenized sample was taken.
2) The sample was deposited in columns with different mixtures of water and zinc sulfate with densities of up to 3 g·cm-3, which allowed only the metallic material to precipitate from the ashes. This density value was taken as a value greater than the relative density of the fly ash normally produced at an industrial level to guarantee the preferential precipitation of the metallic material (Robeck & Huo, 2016).
3) The precipitated and floated material was visually inspected, cleaned by ultrasound in acetone, and subsequently dried at 115° C during 6h.
Taking as a reference the initial metallic content present in the ash samples, these were taken to three gravimetric classification methods: 1) Wilfley table and JIG screen as a wet gravimetric separation medium and mechanical sieving in a sieve as a concentration mechanism in a medium dry. The latter was carried out before and after a milling process. The ashes treated in humid media were carried out using a pulp of 20% solids and a flow rate of 0.5 L·min-1 of water in both cases (Veetil et al., 2014).
The recovery of the metallic material by sieve was carried out using an inclination angle of 10° with a capacity of 25 kg·min-1. The samples were worked with and without previous grinding treatment. The grinding process was developed in a ball mill for 15 minutes at 40 RPM. Subsequently, the samples were dried at 115°C for 6h to ensure the removal of moisture absorbed by the environment before undergoing the gravimetric separation process on a sieve. Each of the trials had 4 replications.
3. Results and discussion
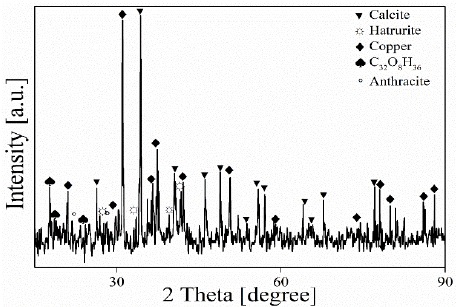
The pyrolysis reactor used in this study was filled with 230kg of WEEE + 20kg of limestone and 80.5kg of ashes were obtained (32.2% of the initial load). The ashes obtained were characterized by X-ray diffraction (XRD). The results obtained are presented in Figure 2. The metallic copper content was estimated at 2.1% by weight using Rietvel analysis, including 47.5% calcite, and 5.8% hatrurite phases that come from the limestone added during the pyrolysis process. The combustion process of the insulation that covers the copper wires generated combustion gases, oils, and solid materials such as anthracite (15%) and other organic compounds (C32O8H36 = 29.2%).
The characterization of the ash sample XRD using the powder technique allowed to establish the presence of copper and metallic copper oxides within the ash. However, to estimate the weight percentage of the metal content in the sample, only the physicochemical separation process was considered. The data obtained shows that the metallic content was 7.1% by weight, i.e., 71 g per kg of ash produced. Likewise, this process determined the relative density of the ash without metallic content. The calculation was made by measuring the initial volume occupied by ashes free of a metallic content and previously dried. Subsequently, the sample was weighed on an analytical balance (measured 3 times) to determine the relative density of the ash. These data allowed obtaining a relative density value of 1.3 ± 0.27 g·cm-3. The relative density found is within the range of the ashes presented in other processes and corroborates the application of hard water with a density of 3 g·cm-3 for the removal of a metallic material within the ashes (Cedex, 2011; Robeck & Huo, 2016).
Due to this, the weight percentage of metal obtained by the selective separation method is taken into account for measurement and comparison during the recovery processes of metallic material in the ash by different gravimetric methods.
Figure 3 shows the XRD pattern of the metallic material collected from the ashes and subsequently melted. The diffraction pattern shows that the composition of the metallic material is copper of the space group Fm-3m (Smura et al., 2011). This indicates that there are no contaminants in the final product, verifying the effectiveness of the process.
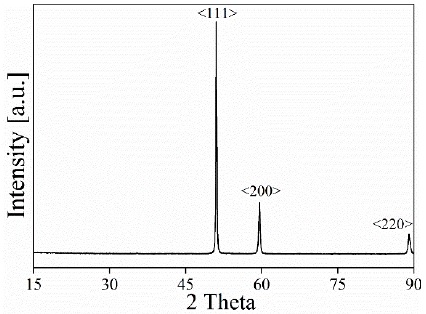
Figure 3 X-ray diffraction pattern of copper recovered from the ashes produced in pyrolysis processes.
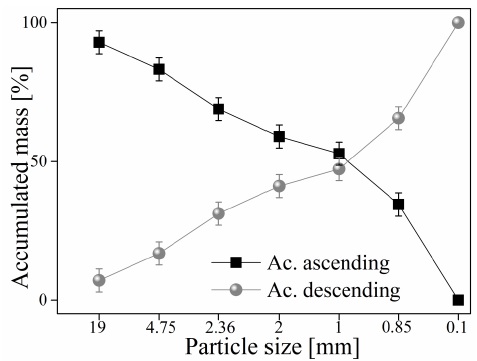
The granulometric analysis carried out on the ashes shows a tendency to generate particles with a size of 1 ± 0.12 mm (50% of the total mass) during the pyrolysis process, favoring the recovery of copper due to the specific density of the ashes worked. The granulometric analysis of Figure 4 shows that approximately 80% of the accumulated decrease has a particle size of less than 5 mm (D4). As a consequence of the decrease in the particle size, the release of copper contained in the accumulated ash is favored, thus allowing the treatment of solid waste (Wills, 2016).
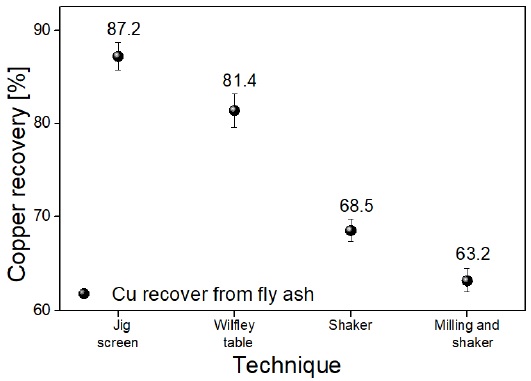
Figure 5 shows the percentages of metallic material recovered in each of the methods used. The recovery percentage was based on the initial content of metallic material present in the ash samples, i.e. 7.1% by weight. From Figure 5, it was observed that in the methods worked in a humid environment they presented a higher recovery of copper. The best result was obtained by using a JIG sieve, where it was possible to recover approximately 87.2% by weight of metallic content present in the ashes.
The recovery of metallic material using the Wilfley table was about 7% less than obtained using the JIG sieve. However, the recovery percentage was higher than that obtained by dry separation methods. The samples worked in a dry environment inside the sieve showed a low recovery percentage, presenting a negative influence in the application of a grinding process before the concentration process. Thus, it was obtained a recovery degree of 63.2%; 5.3% less metallic material than that obtained by applying a screen to ash samples without a previous grinding process. The management of a system in a humid environment allows the buoyancy and greater ease of movement of the particles with a density lower than that of water. Likewise, the delay and stagnation of the metallic pieces with a higher density is favored, recovering it among the ashes.
The management of the gravimetric concentration processes using a vertical bed favored the concentration process of this type of material, taking advantage of the significant difference in the densities between the ashes and the metallic material (Lai & Lu, 2003). The low density of the ash and the management of a vertical water bed, i.e., the use of a system in a humid environment, contributed to an almost total separation of the metal of interest from the undesirable material (Ambrós, Cazacliu, 2016; Ambros, Sampaio, et al., 2016).
The use of a JIG concentration equipment generates a stratification pattern that consists of dividing the bed into three layers: 1) a lower layer filled with the metal extracted from the ashes, as a high-density material; 2) an intermediate layer consisting of a mixture of ash with high specific density and small pieces of copper joined together and; 3) a top layer of low-density ash. However, the use of two types of material in a vertical bed, with a high difference in specific density, favors the absence of the second layer within the JIG sieve (Rojas-Arias et al., 2018).
Figure 6 shows the variation between the percentage of copper recovered and the recovery time. It is observed that an increasing time is required inside the Wilfley table compared to the JIG screen to recover the same weight percent. Likewise, the application of the sieve shows a low performance in the recovery of the material that, although it had a recovery rate similar to that found in the Wilfley table during the first 10 min, it was decreasing periodically until obtaining about 63% and 68 % with and without the application of a previous grinding process, respectively.
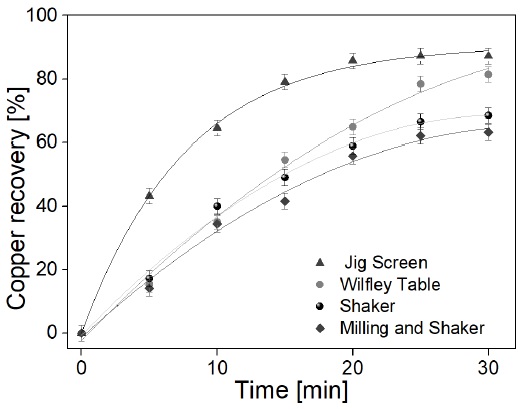
Figure 6 Percentage of the ratio of recovered copper / allotted time of the process in the different gravimetric concentration methods. The curves show the variation of copper recovery (in percentage) at different time intervals.
When using the sieve, a longer time is required for the recovery of the metal contained in the ashes, also increasing the operating time of the process when the sample was previously subjected to grinding processes, in both cases because the material must travel a greater distance for material recovery. It was found a similar behavior to the JIG sieve in the Wilfley table, where a lower degree of concentration per time was obtained in the latter (Gülsoy & Gülcan, 2018).
The handling of a JIG screen using a liquid medium, facilitates the processing of ash of various morphologies and sizes to the use of the Wilfley table and the mechanical screen, which require specific sizes of particles or a similar morphology within the system. This generates a limitation during the recovery process of the metal present within the ashes produced by pyrolysis (Ambrós, Cazacliu, 2016; Ambros, Sampaio, et al., 2016). The morphology of the metallic material and the ash particles are important factors that prevent an optimal recovery of the metal within the Wilfley tables (Legorreta-García et al., 2010).
4. Conclusions
The comparison between three methods of gravimetric concentration for the recovery of copper in the ash produced in pyrometallurgical processes for the calcination of electronic waste was presented.
Employing the presented physicochemical procedure, it was established that the ashes had 7.1% by weight of metallic content, which by characterization by XRD was confirmed as metallic copper. When performing the separation processes by gravimetric concentration, the highest efficiency was obtained within the JIG screen with 87.2% copper recovery by weight. The use of a vertical bed of water allowed an efficient stratification of the material, achieving recoveries up to 80% in 16 minutes for the JIG screen. The morphology of the particles will be a factor to study in future works to determine the behavior in gravimetric systems dependent on it, such as the Wilfley table.











 text new page (beta)
text new page (beta)