1. INTRODUCTION
Water is vital for the survival of earth living beings; and for humans, it has multiple uses, among which drinking water stands out. It must have healthy characteristics to be intake; in particular, it must not contain bacteria that can cause diseases. The bacteria have a great adaptation facility practically to any habitat, and some of them are resistant to high temperatures, extreme dryness, or high humidity (Acquaotta, Ardissino, Fratianni & Perrone, 2017). Several methods of bacteria inactivation have been developed. However, at present, work continues on technologies that can inactivate various microorganisms to offer an adequate quality of water for human consumption (Burns et al., 2012; Murray et al., 2008; Verma, Gupta & Gupta, 2016).
For many years, the classical method of bacteria inactivation has been chlorine addition to water, because it is an efficient disinfectant in the inactivation of bacteria, it is an efficient disinfectant in the inactivation of bacteria, cheap and has a residual effect, that is, it remains active in the water and with the ability to continue inactivating pathogens (Magbanua, Savant &Truax, 2006; Wu, Zhang, You, Yan, & Li, 2016). However, this element in the presence of organic matter can generate toxic compounds such as Trihalomethanes (THMs) and Haloacetic acids (HAAs). Thus, the use of chlorine is restricted in many countries (Hong et al., 2013). As an alternative to chlorine, ozone has been used (formed by three oxygen atoms). The production of this chemical agent is done mainly by electric discharges, through which a gas (oxygen or air) is passed through an electric field, thus generating the ozone in addition of other chemical species. It is an oxidizing agent capable of inactivating bacteria by oxidation of the deoxyribonucleic acid (DNA) (Levén, Wijnbladh, Tuvesson, Kragelund, & Hallin, 2016; Wittmer et al., 2015), lipids of cell membrane, and intracellular organelles (Hashizume et al., 2014) In addition, also to eliminate color, smell, and taste (Loeb, Thompson, Drago, Takahara, & Baig, 2012). The chemical reaction between water and ozone promotes the generation of reactive oxygen species (ROS) such as oxygen (O+, O-), radicals (O2 •-, •OH) and peroxides (H2O2) (Burns et al., 2012; ) that cause oxidative stress in bacteria due to its highly oxidizing properties. ROS contain oxygen atoms in their chemical structure that present a wide lifetime range from nanoseconds to hours. ROS are typically generated using photolysis and energy transfer reactions. These processes generate •OH radicals, which are chemical species with redox potential in the range of 1.9 - 2.85 V (Wardman, 2007), which is higher than the ozone redox potential. The presence of •OH has a fundamental role in the elimination of bacteria since it causes physical destruction of the cytoplasmic membrane by oxidation (Burns et al., 2012; Uhm, Choi, Cho & Hwang, 2013).
Likewise, ultraviolet (UV) radiation can inactivate bacteria, viruses, bacterial spores, and parasites. It does neither alter the physical properties of water, nor produce disinfection byproducts because no toxic compounds are added, but it is believed that this method may have a bacteriostatic effect or a repair of the microorganisms allowing again their population (Koivunen & Heinonen-Tanski, 2005). However, this characteristic depends on the type of water to be treated. This method of disinfection acts causing disturbances in the genetic material (DNA) of the bacteria, which prevents their reproduction. The sufficient wavelength to inactivate the E. coli bacterium is in the region UV-C within a range of 200-280 nanometers (nm) with a higher effect at approximately 260 nm (Magbanua, et al., 2006) in liquid. Additionally, other studies inactivate bacteria in the 380-480 nm range (Tomb et al., 2018). Another method of inactivation is based on the use of TiO2, which is used as a catalyst and has excellent potential for the disinfection and inactivation of harmful pathogens (Reddy, Kavitha, Reddy, & Kim, 2017). Besides, research is being carried out using the physicochemical and photocatalytic mechanisms of metal oxides (Lebedev, Anariba, Tan, Li, & Wu, 2018). Other procedures that are being carried out in bacterial inactivation, among others the E. coli, are employing ozone and photo-assisted disinfection technologies (Gomes, Matos, Gmurek, Quinta-Ferreira, & Martins, 2019).
According to the above, ozone in sequential combination with UV should be an option as a disinfection method, either on surfaces (Kumari et al., 2017) or water (Fang et al., 2014), indicated to be more effective in killing microorganisms than ozone or UV individually. An additional advantage, is the transformation of ozone back into oxygen, without leaving any harmful chemical residue in the water, therefore avoiding the generation of toxic waste (Murray et al., 2008; Karaca & Velioglu, 2014). So that, this technology is expected to become more widespread because of their advantages against other methods as chemical, with public health effects (Magbanua et al., 2006), or catalysis with long exposure periods for achieving reasonable results of inactivation.
In this study, the results of the inactivation of E. coli bacteria in water using ozone and UV radiation are presented. After a treatment time of three minutes, it was achieved bacteria inactivation percentages of 99.32% from an initial concentration of 1.45×103 bacteria/mL inoculated in a vessel containing 500 mL of water which is associated to a continuous flow disinfection system.
2. EXPERIMENTATION
2.1 EXPERIMENTAL SETUP
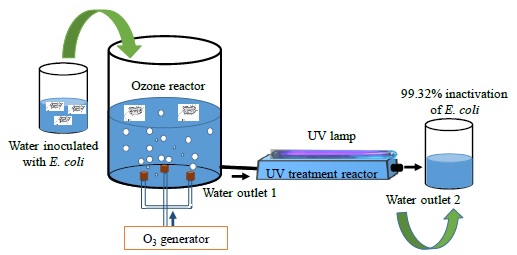
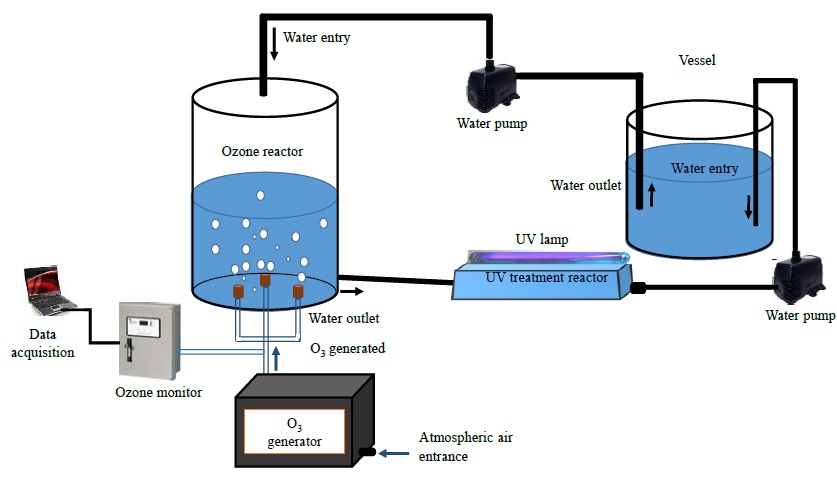
Figure 1 shows the experimental diagram for the inactivation of E. coli bacteria in water employing ozone and UV radiation. This setup is mainly composed of a cylindrical glass reactor with a volume of ~ 1178 cm3. The lid and the base of this reactor were made of nylamid, and has two inlets and outlets, one pair of them for water and the other pair for ozone. The last is produced by an ozone generator and injected using three diffusers placed at 120° from each other at the base of the reactor and where the precursor gas is the ambient air. This system has an energy consumption of 10 W. Moreover, a shortwave ultraviolet radiation (UV-C) generated by a 12 W low-pressure mercury lamp made of quartz is radiated to the UV treatment reactor through a quartz cap located at the top, where the distance from the UV lamp to the surface of the water is ~ 2 cm. This last reactor has a rectangular prism shape rounded at the edges built in glass. It has a volume ~ 550 cm3, an inlet and an outlet of water. Meanwhile, the liquid is pumped with an AC water pump from the vessel and by gravity, the water attains the UV treatment reactor and, finally, the liquid is propelled again to the vessel with another pump. This arrangement represents a closed-loop circulating water circuit and when all the water volume is deposited in the vessel, a treatment cycle was done.
The experiment consisted of using 500 mL of sterilized and inoculated water with a concentration of the order of 103 bacteria/mL, which is deposited in the container, then pumped to the treatment reactor. During water treatment at the first reactor outlet, a sample is acquired to perform the microbiological analysis while a second sample is taken at the outlet of the UV radiation reactor to perform the corresponding microbiological analysis.
Radiation energy per volume (dose) can calculate per unit volume of the liquid to be processed to express the dose (D) in J/L or J/mL as follows (Keyser, Műller, Cilliers, Nel, & Gouws, 2018):
where P is the maximum power of the UV lamp in watts, and q is the volumetric flow of the liquid in L/s or mL/s.
2.2 CELL CULTURE
To carry the study out the E. coli American Type Culture Collection (ATCC) 8739 bacterium was used. It was incubated in 5 mL of Luria-Bertani (LB) nutrient medium for 20 hours at a temperature of 37° C. After incubation, the nutrient medium was withdrawn from the culture tube using a Labnet brand centrifuge operating at 5000 rpm for 10 minutes. After that, the resulting culture was suspended in 5 mL of sterilized water. It was made successive dilutions from this solution to reduce the concentration, until being able to count the number of bacteria employing a Carl Zeiss brand phase contrast microscope and a Neubauer chamber. Then a concentration of the order of 103 bacteria/mL was inoculated in 500 mL of water and deposited in a vessel to start the treatment.
2.3 MICROBIOLOGICAL ANALYSIS
The water inoculated with bacteria E. coli and subsequently treated by ozone and UV radiation phases were subjected to a microbiological analysis to determine the results of bacteria deactivation. Afterward, three 0.1 mL aliquots obtained from treated samples were placed on previously prepared Petri dishes with LB solid nutrient medium, later dispersed and incubated at a temperature of 37° C for 20 hours.
The reference sample was taken from the vessel at the beginning of the water treatment process. Considering that each viable bacterium forms a colony (CFU), the colonies were counted after incubation using a SOLBAT colonies counter. This process is applied to the treated samples, so after counting each one of the CFUs, the number of surviving bacteria (NBS) in CFU/mL is obtained from the following expression:
Where NC is the number of counted colonies (in CFU) and VMU is the volume of the used sample (0.1 mL).
While the inactivation percentage is calculated as:
where: NO is the number of counted colonies (CFU/mL) of the reference sample.
3. RESULTS
3.1 OZONE CHARACTERIZATION
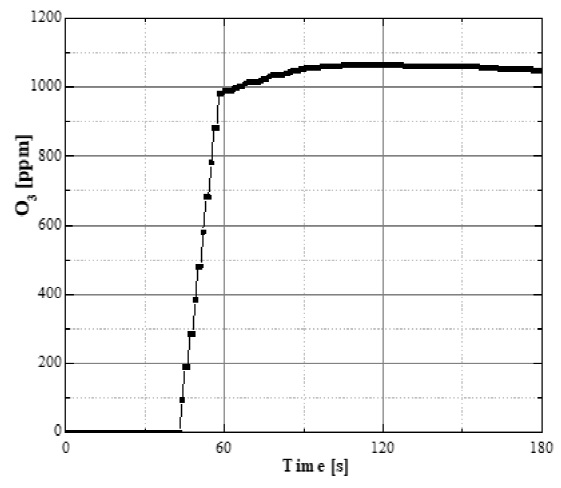
The characterization of the ozone was made at the entrance of the treatment reactor using an ozone monitor (Teledyne Instruments 460L) through an interface to a personal computer using a data acquisition system. Results were obtained by sampling every second and plotted later. The amount of ozone supplied to the reactor at steady-state (approximately 60 s after ozone generator is turned-on) was in the range of 1000-1100 ppm. Figure 2 shows the resulting ozone concentration at the treatment reactor inlet.
3.2 CHARACTERIZATION OF UV RADIATION
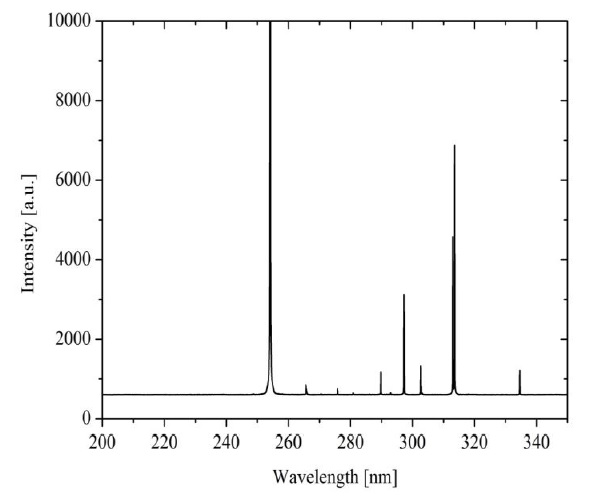
It can be observed in Figure 3 obtained optical emission spectrum from the UV lamp used. It was acquired in a wavelength range of 200-350 nm, showing characteristic peaks over wavelengths of 265-335 nm with peak emission at 254 nm, which corresponds to the typical wavelength where bacteria show susceptibility to inactivation (Coohill & Sagripanti, 2008). The characterization was achieved using a Jaz OceanOpticsTM optical emission spectroscopy (OES) with an optical resolution of 0.3 nm. The power of the lamp was 12 W, and the volumetric flow was 8.33 mL/s, then, using (1) the estimated UV radiation energy per volume was 1.44 J/mL.
3.3 INACTIVATION OF E. COLI BACTERIA
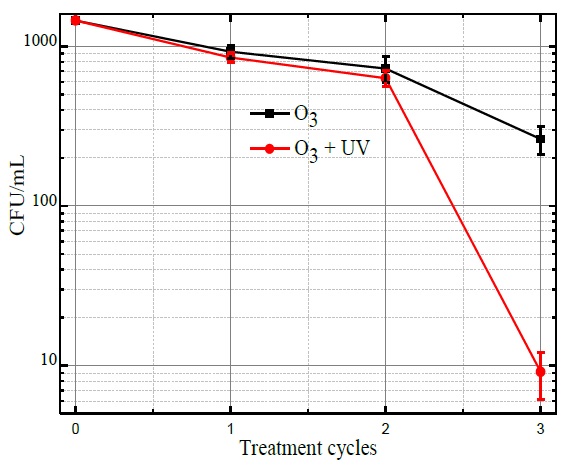
Figure 4 shows the qualitative results of E. coli bacteria on agar after 20 hours of incubation. Figure 4.a corresponds to the reference sample representing a concentration of 1.45×103 CFU/mL. Figure 4.b and Figure 4.c show the results after the first treatment cycle for treatments of ozonation and ozonation and UV radiation, respectively. Figure 4.d and Figure 4.e depict the results after the second treatment cycle for treatments of ozonation and ozonation and UV radiation respectively, and, Figure 4.f and Figure 4.g correspond to the results after the third treatment cycle for both applied treatments.
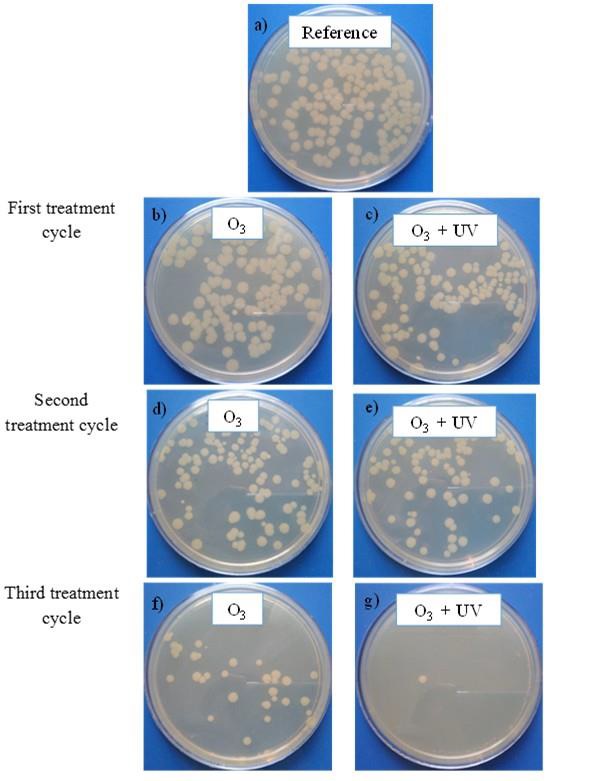
Fig. 4 Inactivation of E. coli using ozone and UV radiation. Images of experimentalresults after 20 h incubation.
The inactivation of the E. coli bacteria was evaluated from the comparison of CFU/mL of the reference sample Figure 4(a) concerning the CFU/mL after performing the combined ozonation and UV radiation treatments. From the results shown in Figure 5, it is observed that the average initial concentration about 1.45×103 CFU/mL, after the first treatment cycle bacteria concentration was reduced to 920 CFU/mL with ozone treatment and to 860 CFU/mL with ozone treatment plus UV radiation. Afterward, the water was recirculated, and after the second treatment cycle, bacteria concentration was reduced to 760 CFU/mL with ozone and to 620 CFU/mL with ozonation coupled to UV radiation. Finally, the resulting bacteria concentration at the end of the third cycle was 250 CFU/mL with ozone and 10 CFU/mL applying ozone and UV radiation.
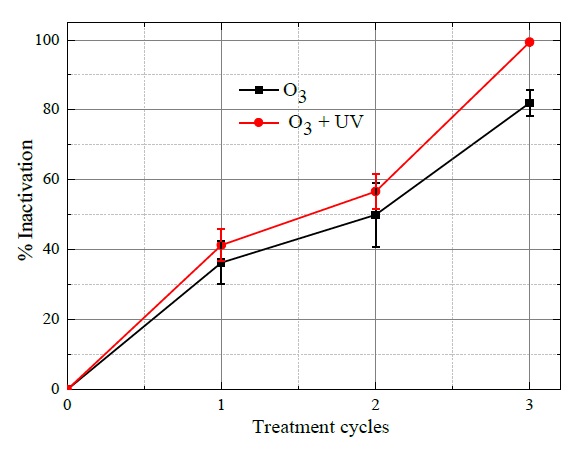
The percentages of E. coli bacteria inactivation are depicted in Figure 6, where it can be observed that as increasing the number of treatment cycles, the higher percentage of bacteria inactivation was attained. Therefore, at the end of the first treatment cycle, the obtained bacteria inactivation with O3 and combined ozone with UV radiation were respectively 37% and 41%. While after the second treatment cycle, the resulting deactivation efficiencies were 48% with O3 and 58% with O3 and UV radiation. Finally, the higher efficiency percentages were attained the third treatment cycle (180 s), afterward with 83% supplying O3 and 99.32% combining ozone and UV radiation. These results indicate that although both processes can be used in the inactivation of E. coli, ozone with UV has the potential to enhance the disinfection process.
4. DISCUSSION
When ozone is applied to a liquid, a large number of bubbles are generated as has been observed by other authors (Sumikura, Hidaka, Murakami, Nobutomo, & Murakami, 2007; Mishra et al., 2017). In our case, the ozone supply into the ozonation reactor promotes a considerable amount of bubbles and microbubbles at the whole volume of contained water, achieving a high and uniform contact between generated ROS with bacteria inoculated in water. ROS can be produced in this process by the next chemical reactions. Ozone is broken down into atomic and diatomic oxygen by the reaction:
Atomic oxygen can take as a vehicle the generated bubbles and has a more effective interaction with the bacteria cellular membrane promoting their lipid oxidation, abstracting hydrogen from membrane lipids and proteins to form peroxides (Wang, Libardo, Angeles-Boza, & Pellois, 2017). Also, when atomic oxygen reacts with water, it generates •OH radical (Karaca & Velioglu, 2014) which has a considerable redox potential in the range of 1.9 - 2.85 V, which can interact directly with bacteria. ROS such as the •OH, as observed in previous studies (Rodríguez-Mendez et al., 2013), could also be the cause of a chemical attack on the bacteria, such as ozone that generates oxidative stress on the cellular membrane (Karaca & Velioglu, 2014; Uhm, et al., 2013). The •OH is generated by:
In the first instance, this mechanism could be responsible for the inactivation effectiveness against E. coli by means ozone. Furthermore, ozonation treatment is complemented with UV radiation, because it generates •OH radicals in the presence of water at λ = 200-280 nm. As it is established in the next reaction:
In addition to chemical oxidation processes with the membrane cells produced by the generated of chemical species with a more significant redox potential such as: O, O3, •OH; ones UV radiation can penetrate the bacteria making a muddle of its genetic material which causes the inactivation of E. coli bacteria (The above mentioned could explain the results shown in Fig. 5. and Fig. 6, where the effect of the O3/UV treatment (similar to O3 until the second treatment cycle) against E. coli from the third treatment is evident. As can be seen, in overall water treatment of three cycles (180 seconds), E. coli were inactivated much more rapidly with the combined ozonation and UV treatments as compared to the O3 treatment, corresponding to more than an order of magnitude of inactivation higher than with O3 alone. These characteristics made this a low cost and effective method to inactivate bacteria in water flowing continuously with high bacterial inactivation efficiency, in relatively short treatment times.
5. CONCLUSIONS
The here proposed treatment system turned out to be very efficient, obtaining a 99.32% inactivation of E. coli bacteria at a concentration of 1.45×103 CFU/mL. Two inactivation techniques were applied in the treatment system: the first one based on the injection of ozone at the base of a cylindrical reactor and the second one determined by UV radiation exposure in a rectangular reactor, achieving a satisfactory efficiency magnitude. It must be noted that results mainly are determined by the biological interaction between bacteria structure and the generated chemical species and applied UV radiation. This methodology does not make use of supplement chemical products and consumes ambient air as a primary resource, making this proposal harmless to the environment.











 nueva página del texto (beta)
nueva página del texto (beta)