1. INTRODUCTION
Melanoma is a very dangerous skin cancer due to its ability to metastasize and acquire chemo- or radioresistance (Vermeylen et al., 2016). The incidence of metastatic melanoma has been increasing over the past 35 years (Gray-Schopfer, Wellbrock, & C Marais, 2007), so is important, along with early detection, the search for new treatments. The B16 murine cell line has been used extensively as a model for the study of this type of cancer.
In physics, plasma is defined as a partially ionized gas containing free charge carriers (ions and electrons), active radicals, excited molecules and ultraviolet emissions (Kogelschatz, 2003). In the laboratory, thermal (quasi-equilibrium) and non-thermal (out of equilibrium) are produced at different pressures, depending on the required technological application. In the last years, non-thermal plasma has been used for surface (Nehra, Kumar, & Dwivedi, 2008; Laroussi, 1996; Lerouge, Wertheimer, & Yahia, 2001) and heat sensitive medical material sterilization (Fridman, 2008), wound healing and tissue sterilization (Kieft, Darios, Rooks, & Stoffels, 2005; Laroussi, 2009), blood coagulation (Fridman, 2008) skin treatments (Fridman et al., 2007; Fridman, 2008), bacteria inactivation (Gallagher et al., 2007; García-Alcantara et al., 2012; Laroussi, Richardson, & Dobbs, 2002; Martines et al., 2009; Moisan et al., 2001; Sladek, Stoffels, Walraven, Tielbeek & Koolhoven, 2004;) and functional and structural modification of cancer cells (Fridman, 2008; Stoffels, 2003; Zimheld, Zucker, Zimheld, Zucker, DiSanto, Berezney, & Etemadi, 2010).
It has been proposed that the reactive oxygen/nitrogen species (RONS) generated by plasma can disrupt cell membrane and attack several biomolecules, including DNA (Yan, Sherman, & Keidar, 2017). Indeed, experiments made with a bacterial strain defective in a protective system to oxidizing agents showed that it was more sensitive to plasma than a wild-type strain (Garcia-Alcantara et al., 2013). Accordingly, the effect of non-thermal plasma upon the genetic material has been demonstrated before, either in naked DNA (Morales-Ramirez et al., 2013) or in living cells (García-Alcantara et al., 2013). To date, several assays have been used to assess DNA damage, such as plasmid breakage, micronuclei induction or phosphorylated histone H2AX detection in a wide range of cell types (Alkawareek et al., 2014; Kim, Kim, & Lee, 2010; Kluge et al., 2016).
The purpose of the present work is to evaluate the sensitivity of the murine melanoma cell line B16 to a helium-generated plasma needle exposure.
2. METHODOLOGY
2.1 CELL CULTURES
Murine melanoma cells (B16) and 3T3 fibroblasts were grown at 37°C and 5% CO2 atmosphere, in minimal essential medium (MEM) supplemented with 10% FBS, 1% antibiotics (penicillin, streptomycin and amphotericin) and 0.3 mg/ml L-glutamine until subconfluent phase was reached. Cultures growth was monitored using an inverted microscope. Cells were harvested by trypsinization with a 0.01% trypsin-EDTA solution, washed twice with Hanks Balanced Saline Solution (HBSS) and further incubated for at least one hour in MEM at 37°C to recover prior plasma exposure Human lymphocytes were obtained from blood samples drawn by venipuncture from healthy donors, mixed 1:1 with HBSS, gently poured on top of an equal volume of Ficoll Hypaque and centrifugated for 10 minutes at 2,000 rpm. The inter-phase ring of nucleated cells was then collected and washed twice with HBSS, resuspended in RPMI-1640 supplemented with 10% FBS and placed again at 37°C and a 5% CO2 atmosphere for at least one hour.
2.2 PLASMA EXPOSURES
Plasma generator is based on a radiofrequency 13.56 MHz commercial power source linked to a homemade PI matching box, which in turns supplies a punctual non-thermal plasma reactor (Pérez et al., 2008). The last has a coaxial configuration built with an energized copper filament surrounded by an isolating ceramic, both linked to a cylindrical Nylamid SL frame with 8 cm of length. This structure has a gas inlet and a female micro RF connector. A 1 mm visible non-thermal plasma discharge is produced at the head of copper filament which is encircled by an external thin copper grounded electrode. When 8 W were applied from the RF power source to the load (mode load power), it was generated a power density along the radiofrequency cable and the reactor of 0.2416 W/cm2. The three cell types were resuspended in HBSS and then 200 µl aliquots were distributed in a 96-microwell plate and exposed to plasma generated by a flow of 0.7 LPM of helium electrically excited by means of a 13.56 MHz radiofrequency generator at a power of 8 W. The plasma reactor outlet was kept at a distance of 2 mm from the liquid surface.
2.3 CYTOTOXICITY
Cell death was evaluated according to the method of Strauss (1991). After treatments, cells were stained with a 1:1 fluorescein diacetate (80 µg/ml) and ethidium bromide (50 µg/ml) solution and observed afterward under a Hund Wetzlar fluorescence microscope with an excitation filter of 488 nm (blue light). Living cells are stained in green while dead cells are stained in red. A minimum of 100 cells per treatment were scored and all the experiments were repeated at least three times. Survival was calculated by dividing the number of living cells by the total number of cells.
2.4 GENOTOXICITY
Genotoxicity was evaluated using the comet assay (Tice et al., 2000). After plasma exposure, cell suspensions were mixed with an equal volume of 1% low melting point agarose (LMPA) and immediately 80μl aliquots were dispensed on top of fully frosted slides, let to solidify for 5 minutes and then immerse in cold lysis solution (2.5M NaCl, 100mM EDTA, 10mM Tris, 1% SDS, 10% DMSO, 1% TRITON X100, pH10) for at least an hour. Afterward, slides were transferred to an electrophoresis cell filled with electrophoresis solution (0.3M NaHO, 0.1 mM Na2EDTA) for 20 minutes to allow DNA unwinding and thereafter a current was applied (20 V, 300 mA, 20 minutes). Slides were rinsed twice with neutralizing buffer (0.4M Tris-HCl, pH 7.5), stained with 60 µl of a 20 µg/ml ethidium bromide solution and observed under an epifluorescence microscope. Images (at least 100 per dose) were scored by the Comet Assay IV Analyzer (Perceptive Instruments Inc.). All the experiments were repeated no less than three times.
3. RESULTS AND DISCUSSION
In the conditions stated above, the non-thermal plasma generator delivered the following energy density (Table 1):
Table 1 Energy delivered at different exposition times.
| Time (seconds) | Equivalent Energy Density
(J/cm2) |
| 15 | 3.62 |
| 30 | 7.25 |
| 45 | 10.87 |
| 60 | 14.50 |
The results show that the exposure to the non-thermal plasma produced both cytotoxicity and genotoxicity upon cells in a dose-dependent manner. Overall, the effect of plasma was higher in melanoma cells than in human lymphocytes or fibroblasts. The idea to use lymphocytes was to compare the effect of non-thermal plasma upon proliferative and no proliferative cells. We decided to use human lymphocytes because of the volume required and the ease to obtain them.
As shown in Figure 1, fibroblasts are less sensitive to plasma exposure compared with lymphocytes and B16 cells. Lymphocytes were slightly more sensitive than fibroblasts, with about 50% of survival at the higher dose, while B16 cells had the highest sensitivity to non-thermal plasma, showing a cellular death above 90% at the higher dose.
B16 cells suffer higher genetic damage compared with fibroblasts and lymphocytes as the energy deposited in the cells rises, as shown in Figures 2 and 3. Nevertheless, fibroblasts and lymphocytes show a similar behavior, while the DNA breakage in both is lower than the one observed in B16 cells. Overall, all three cell lines show an increase in DNA fragmentation as the dose rises.
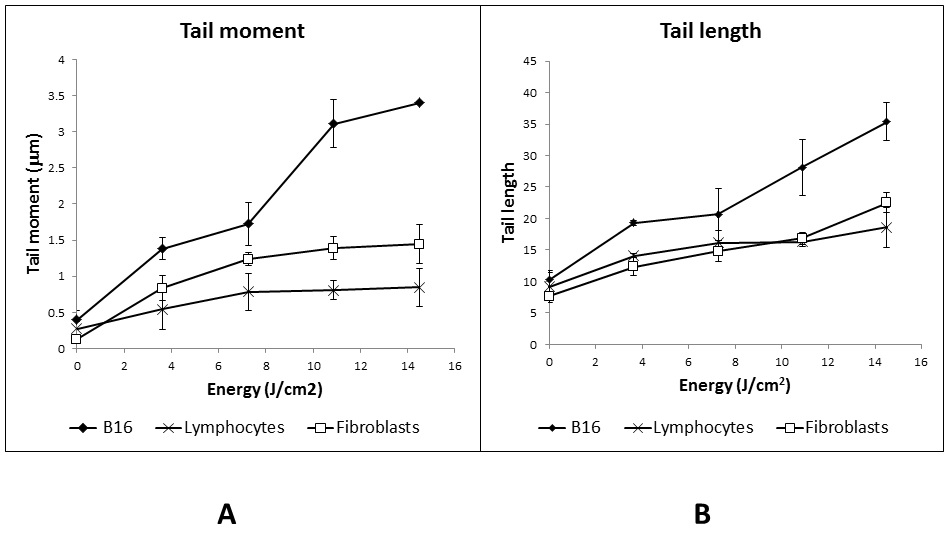
Fig 2 DNA fragmentation in cells exposed to non-thermal plasma, assessed by the comet assay. A) Tail Moment; B) Tail Length.
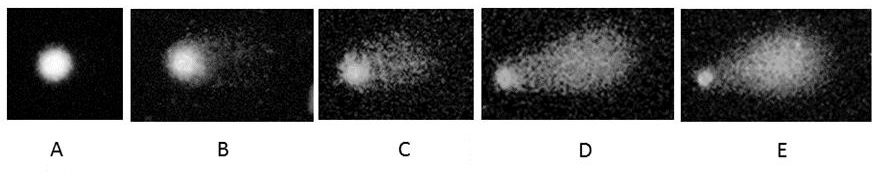
Fig. 3 Images of the different comets found according to the dose. A) 0; B) 15; C) 30; D) 45; E) 60.
As stated above, when plasma is generated intramolecular collisions occur resulting in the emergence of ions and free radicals. The interaction with air results in a partial dissociation and ionization of O2, N2 and H2O, generating reactive oxygen species (ie. O3, H2O2, OH or even N), oxides of nitrogen (ie. NO, NO2, N2O, ONOO), nitrous and nitric acid (HNO3, HNO2) (Yan et al., 2017). All of these products can attack different parts of the cell, such as lipids of the membrane (Van der Paal et al., 2016), proteins in general, mitochondria, as well as genetic material (Kalghatg et al., 2011; Morales-Ramirez et al., 2013). Plasma also generates UV radiation (Stoffels, Sakiyama, & Grave, 2008), especially UVB, which can produce cyclobutane pyrimidine dimers (CPD) and pyrimidine (6-4) pyrimidone photoproducts (6-4PP) upon DNA, which arrest the replication fork and hence could result in cellular death or apoptosis (You et al., 2001).
According to survival results, non-thermal plasma produces higher cytotoxicity on B16 cells compared to lymphocytes or fibroblasts, maybe because this cell line is in active replication and according to the Bergonié and Tribondeau law’s, cells with high metabolic rates or in constant replication are more sensitive. In fact, this is the reason why lymphocytes (which are in a G0 state) were included in the present work. Although fibroblasts, as well as B16 cells, are in constant replication, it is possible that the presence of peroxisomes could protect these cells to the action of RONS, resulting in a higher survival percentage (Brun et al., 2014). Indeed, it has been demonstrated that low levels of RONS increase the migration and proliferation of fibroblast as part of tissue regeneration processes (Gurtner, Werner, Barrandon, & Longaker 2008), promoting an increase in the number of intracellular peroxisomes.
Genetic damage on the three cell lines was evaluated by comet assay. Results show a proportional increase of DNA fragmentation as the dose increases. The results data indicate that CAP breaks the genetic material, most probably due to the RONs generation. Furthermore, DNA damage generated by UV radiation could result in additional breakage due to the repair mechanisms. Once again, B16 cells showed a more severe DNA fragmentation most probably because of their accelerated metabolism. Fibroblasts appear to be as resistant to the genotoxic effect of plasma as lymphocytes, although they are in active replication. Once more, it is possible that the presence of peroxisomes protects the cells from the RONS, resulting in a lower DNA fragmentation. Additionally, recent studies demonstrated that many melanoma cell lines are defective in cytoglobin (Fujita et al., 2014), a new member of the globin family that has been proposed to have a potential role in reactive oxygen species (ROS) detoxification (Fordel et al., 2006; Hodges, Innocent, Dhanda, & Graham, 2008; Nishi et al., 2011). Indeed, recent results show a strong correlation between the expression level of Cygb, the intracellular ROS concentration and the sensitivity of melanoma cell lines towards non-thermal plasma treatment (De Backer et al., 2018).
4. CONCLUSIONS
Exposition to non-thermal plasma causes cytotoxicity and genotoxicity on the B16 cell in a dose-dependent way. It was observed that the lethality produced by this kind of plasma was higher in melanoma mouse cells compared with lymphocytes and fibroblasts. Accordingly, B16 cells showed higher levels of DNA fragmentation by this agent. Although in the present work only cyto- and genotoxicity data are presented, is important to underline that actually there are differences in the effect produced by non-thermal plasma on normal and melanoma cells, which is a key melanoma cells, which is a key feature in the safety treatment of cancer patients. The results suggest that non-thermal plasma has the potential to become a good alternative for skin cancer treatment.











 nova página do texto(beta)
nova página do texto(beta)



