1. Introduction
Biodiesel, an alternative to fossil fuels, is basically a mixture of Fatty Acids Alkyl Esters obtained from renewable sources, mainly by transesterification of either vegetable oils or animal fats. Transesterification is a chemical reaction between large branched triglycerides (TGs) or fatty acids and an alcohol (most often methanol) under the presence of a catalyst and temperature to produce alkyl (most often methyl) esters and glycerol. Transesterification implies a sequence of reversible and consecutive reactions: (1) TGs react with methanol (M) and are converted into diglycerides (DGs), (2) DGs are further converted into monoglycerides (MGs) and (3) MGs are finally transformed into glycerol (G). In each step an ester is produced and therefore three molecules of ester (E) are produced for every reacted molecule of TG. Since reaction proceeds too slow at standard conditions, a catalyst is used in order to accelerate it; being the recommended catalysts either base or acid. When the transesterification of oils with low free fatty acids (FFA) content is to be conducted solid base catalysts are preferred (Lee & Wilson, 2015). Among these catalysts, CaO-based catalysts have been extensively studied and recognized as promising catalysts to conduct the transesterification of vegetable oils at industrial scale recognized as promising catalysts to conduct the transesterification of vegetable oils at industrial scale (Alba-Rubio et al., 2010; Arzamendi, Arguiñarena, Campo, Zabala, & Gandía, 2008; Boey, Ganesan, Maniam, & Khairuddean, 2012; Khemthong et al., 2012; Viola et al., 2012). This is due to CaO exhibiting low solubility in organic compounds, high basicity, no toxicity, easy handling, long useful life, human exposure benign, reusability and low cost and natural availability of precursors, i.e. limestone (Alba-Rubio et al., 2010; Arzamendi et al., 2008; Khemthong et al., 2012). CaO has been demonstrated to be an excellent catalyst not only for pure oils but also for waste oil (Boey et al., 2012; Viola et al., 2012). Also, in the vast existing literature on transesterification catalyzed by CaO, it has been demonstrated that not only the methanol:oil ratio, catalyst loading, temperature, doping, type of alcohol and feedstock impact the attained yield of fatty acids alkyls esters but also the CaO precursor does (Tang, Yan, Shen, Li, & Jeje, 2016). It has been found (Tang et al., 2016) that CaO from calcium carbonate exhibits more active sites and larger surface areas than the oxide from other precursors (acetate, hydroxide and oxalate). CaO catalytic performance is also affected by its combination with other oxides like MgO (Ilgen, 2011; Ilgen & Akin, 2012), ZnO (Alba-Rubio et al., 2010; Lukic et al., 2014; Ngamcharussrivichai, Totarat, & Bunyakiat, 2008) or Al2O3 (Marinković et al., 2017) and doping with metals like Li (Alonso, Mariscal, Granados, & Maireles-Torres, 2009), Zn (Banković-Ilić, Miladinović, Stamenković, & Veljković, 2017) and Na compounds (Luz Martínez et al., 2011). In most of the cases, CaO reagent grade has been used. For the sake of sustainability and economical viability, however, other sources of CaO have been explored. Within these alternatives, eggshells (Risso, Ferraz, Meireles, Fonseca, & Vital, 2018), sea sand (Muciño et al., 2014) and quicklime (Camacho et al., 2016; Miladinović, Krstić, Tasić, Stamenković, & Veljković, 2014) can be found. Quicklime has been recognized as a rather low cost source of CaO and has already been successfully applied in the methanolysis of safflower oil (Camacho et al., 2016), sunflower oil (Miladinović et al., 2014) and rapeseed oil (Masato, Jyu-suke, Yoshiya, & Haruhiko, 2009). The higher activity of quicklime compared to CaO (Camacho et al., 2016) reagent grade and to Li/CaO (Alonso et al., 2009) has already been demonstrated, with safflower and sunflower oil, respectively, though. In this work, quicklime was used to catalyze the transesterification of canola oil and the main objective was to establish a kinetic model that allows not only to attain a further insight into this reaction but also to be able to further design and scale a process involving, both, quicklime and canola oil. It is worth noticing that the quicklime used in this work has also some content of MgO and this has been previously reported (Camacho et al., 2016). Although canola oil is an edible oil, its use as feedstock in biodiesel production is preferred due to its high oil content and its use may increase due to a recent study that demonstrates the production of free glycerol biodiesel from canola oil (Tang et al., 2016). Furthermore, this oil, due to its chain of 18 carbon atoms and an unsaturated bond results in biodiesel with a superior cold flow property (Ilgen, 2012a).
In the context of biodiesel production by canola oil methanolysis, kinetic models have been obtained when dolomite (Ilgen, 2012a) and nanopowder reagent grade CaO (Zhao, Qiu, & Stagg-Williams, 2013) have been used as catalysts. In the former, it was concluded that triglycerides are not chemisorbed and in the latter the best model was concluded to be the one assuming both compounds, methanol and triglycerides, chemisorbed onto the catalytic surface (Langmuir-Hinshelwood-Hougen-Watson approach). Although the quicklime used in this work is basically CaO (Camacho et al., 2016), the textural and basicity properties are different to those of the catalysts assessed in previous works (Zhao et al., 2013). Thus, from the understanding and process design point of view is rather important to establish weather or not both reagents are chemisorbed onto the surface and to establish the kinetic and adsorption parameters when canola oil methanolysis is catalyzed by quicklime, which is a rather low cost heterogeneous catalyst that may help to increase economical viability of biodiesel production process. In order to achieve this aim, different models based on Eley-Rideal (ER) or Langmuir-Hinshelwood-Hougen-Watson (LHHW) mechanisms were assessed in this work based on previous kinetic studies with other catalysts or feedstock (Ilgen, 2012a; Miladinović et al., 2014; Zhao et al., 2013).
2. Materials and methods
2.1 Materials
Food-grade Canola oil was used as raw material and it was purchased from a local grocery store. Its fatty acid composition consisted of palmitic acid (3.5%), stearic acid (1.5%), oleic acid (60.1%), linoleic acid (20.1%), and others (14.8%). Quicklime was purchased from a rural market and characterized in a previous paper (Camacho et al., 2016). Anhydrous methanol (99.96%) was supplied by J.T. Baker. Methanol (99.9%) and heptane (HPLC grade) was purchased from Fermont Co. The gas chromatography reference standard for fatty acid methyl esters was purchased in Supelco. Methyl heptadecanoate was purchased in Sigma Aldrich (puriss. p.a., standard for GC, ≥99.7%) and was used for quantifying total methyl esters.
2.2 Catalyst preparation and characterization
Quicklime was triturated, meshed and recovered between 0.12 mm and 0.84 mm mesh, then calcined at 900 °C for 8 h. This temperature was established by a TGA analysis to be the one at which all CaCO3 is converted and surface water is desorbed. Relatively large exposure to air was avoided in order to prevent contamination. The main crystalline phase of the calcined catalyst was found by XRD to be CaO; however, MgO was also identified. The basicity and base strength were determined by Hammett method ( Hammett & Deyrup, 1932) to be 0.228 mmol/g and 9.8>H_>15, respectively. The details of the catalyst characterization have already been reported in (Camacho et al., 2016) and therefore are not repeated here.
2.3 Reaction procedure
Transesterification reactions were carried out in a 250 mL glass stirred tank reactor with a condenser, baffles and heating and stirring plate. The reaction volume was 100 mL. The assessed variables in this work were the catalyst loading, methanol-to-oil molar ratio and the addition order of raw materials. The response variable at all cases was the FAME content.
When not being the variable under study, the stirring rate, methanol-oil molar ratio, catalyst loading, order of reagents addition, raw materials source and temperature were kept constant at all experiments. The stirring rate was 1000 rpm. Experiments were performed with a methanol-oil molar ratio of 6:1, 9:1 and 12:1.The studied catalyst loading was 2, 3, 6 and 8% (w/w) with respect to the reaction mixture weight, and the reaction time was 5 h at all experiments. The maximum reaction temperature was set at 328 K in order to reduce methanol evaporation without compromising much the energy provided to activate the catalyst and the reaction. It is worth pointing out that the boiling point of methanol is reduced at the atmospheric pressure (558 mmHg) of the place where this research was conducted. The reaction conditions for each experiment are summarized in Table 1. Each experiment was conducted by duplicate. Upon reaction completion, catalyst was separated from the products (glycerol and methyl esters) by centrifugation. Residual methanol was evaporated in a rotavapor (R-215 Buchi Switzerland) under vacuum.
Table 1 Reaction conditions: a summary.
| Code | Molar ratio (Oil: Methanol) | Addition Order | Catalyst loading (w/w)% | Temperature (K) |
|---|---|---|---|---|
| 1 | (1:6) | Methanol-Catalyst | 6% | 328 |
| 2 | (1:9) | Methanol-Catalyst | 6% | 328 |
| 3 | (1:6) | Methanol-Catalyst | 8% | 328 |
| 4 | (1:9) | Methanol-Catalyst | 8% | 328 |
| 5 | (1:12) | Methanol-Catalyst | 8% | 328 |
| 6 | (1:6) | Oil-Catalyst | 6% | 328 |
| 7 | (1:9) | Oil-Catalyst | 8% | 328 |
| 8 | (1:6) | Oil-Catalyst | 8% | 328 |
| 9 | (1:12) | Methanol-Catalyst | 6% | 328 |
| 10 | (1:6) | Methanol-Catalyst | 3% | 328 |
| 11 | (1:6) | Methanol-Catalyst | 2% | 328 |
2.4 Biodiesel characterization
Samples (1.5 mL) were withdrawn every hour from reaction mixture. Samples were cooled down and the catalyst was separated from the products and reactants by centrifugation at 10,000 rpm during 10 min. Then, the residual methanol was evaporated by heating samples. These samples were analysed using a Varian CP-3800 gas chromatograph with flame ionization detector (FID). To quantify the methyl esters content in concordance with norm EN 14103, a capillary column Agilent HP-Innowax column (30 m × 0.32 mm. ×0.25 μm) was used. To measure glycerides the EN-14105 standard was followed (with modification of the injection conditions due to the high initial concentration of glycerides) with a HP-DB5-HT capillary column (15 m x 0.320 mm x 0.10 μm). Helium was used as carrier gas in both analyses.
A Varian 720-ES Series ICP Optica Emission Spectrometer was employed to quantify the amount of Ca and Mg in the resulting biodiesel in the experiments where the methanol:oil ratio was investigated.
2.5 Kinetic modeling
As it is produced in batch mode, the FAME production rate is given by Equation 1,
Where
where n FAME is the number of produced moles of methyl esters (FAME), W is the catalyst mass and r´ is the FAME production rate per mass of catalyst.
In order to establish the kinetic model that best represents the transesterification of canola oil catalyzed by calcined quicklime, two mechanistic approaches were assessed, i.e. Langmuir-Hinshelwood and Eley-Rideal.
In both approaches the following assumptions were made,
The reaction mixture is perfectly mixed so the reagents distribution and concentration are uniform in the whole reaction volume.
At first, the reaction mixture contains only 3 species (methanol, oil and calcined quicklime), two of them are immiscible phases: methanol and oil. As reaction continues, other species like monoglycerides, diglycerides, glycerol and FAMEs are produced, but the concentration of diglycerides and monoglycerides in the liquid phase of the reaction mixture does not present a significant change compared to triglycerides and methyl esters with reaction time, because once they are formed they react quickly with adsorbed methanol onto the catalytic surface until be transformed into methyl esters, so their consumption rates are much faster than that of triglycerides. Thus, the concentrations of monoglycerides and diglycerides are too small and they can be neglected from the kinetic modelling (Galván Muciño et al., 2016).
The contribution to the reaction due to the homogeneously catalyzed methanolysis due to the leached calcium is considered negligible if the catalyst loading is ≥1% (w/w) based on the oil mass. This is the case in this paper.
For each mechanism, several rate equations were deduced depending on the assumed rate-determining step.
The LHHW mechanism was established by assuming that both, methanol (M) and triglycerides (T) are adsorbed onto common active sites and react to produce adsorbed glycerol (G) and adsorbed methyl esters (E). Finally, glycerol and adsorbed methyl esters are desorbed from the catalytic surface. All these steps are reversible. Thus, the rate determining elementary steps and the accordingly established models are presented in Table 2.
Table 2 Kinetic models based on LHHW mechanism.
| Rate limiting step | Model | Simplified Model |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The ER mechanism based models assume the adsorption of methanol (M) on the catalytic surface and then the surface reaction between triglycerides (T) with adsorbed methanol (M) proceeds to form adsorbed glycerol (G) and free methyl esters (E). Finally, adsorbed glycerol is desorbed from the catalytic surface. All these steps are considered reversible. The rate determining elementary steps and the accordingly established models are presented in Table 3.
Table 3 Kinetic models based on ER mechanism
| Rate limiting step | Model | Simplified Model |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
In addition, two other set of kinetic models based on LHHW and ER mechanisms were assessed and these are summarized in Tables 4 and 5, respectively. The difference between the models presented in Tables 4 and 5 and the ones presented in Tables 2 and 3, is that the glycerol adsorption term is assumed small compared to the other adsorption terms.
Table 4 Kinetic models based on LHHW mechanism with weak adsorption of products.
| Rate limiting step | Model | Simplified Model |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- | - |
|
|
|
|
Table 5 Kinetic models based on ER mechanism with weak glycerol adsorption.
| Rate limiting step | Model | Simplified Model |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Although glycerol has the property of attaching to the catalyst and form species known as calcium diglyceroxide CH3-O-Ca-O(OH)2C3H5, this may not deactivate the active sites in catalyst; in fact this specie has been proven to exhibit catalytic activity on the transesterification reaction (Kouzu, Fujimori, Suzuki, Koshi, & Moriyasu, 2017).
Contrast between the different kinetic models was performed considering the physical significance of the estimated parameters and models were discarded if no consistency with experimental data was shown or kinetic parameters with erroneous values, i.e., negative values, too high values or not significantly different from zero at the 95% confidence level. To perform the minimization of the objective function the software POLYMATH 5.1 applying the Levenberg-Marquardt algorithm was utilized.
3. Results and discussion
3.1 Transesterification of canola oil
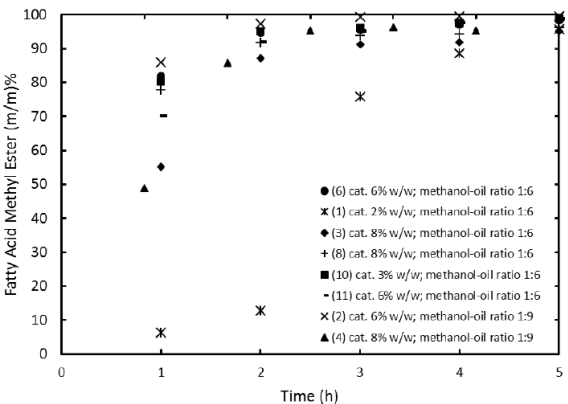
Reaction experiments described in section 2.3 were performed by duplicate and FAME profiles as function of time were established. By comparing the FAME profiles of experiments 1, 3 and 4 (methanol-catalyst mixed at first) versus 6, 7 and 8 (oil-catalyst mixed first) in Figure 1, it can be concluded that the reagents order of addition does not affect the time at which equilibrium is reached (about 5 h). Initial production rate (given by the slope of each profile at zero time) is found to be not significantly dependent on reagents order addition. This suggests canola oil transesterification catalyzed by quicklime may not follow the methanol adsorption as the rate-determining step as observed in the transesterification of safflower oil catalyzed by K2O/NaX [15].
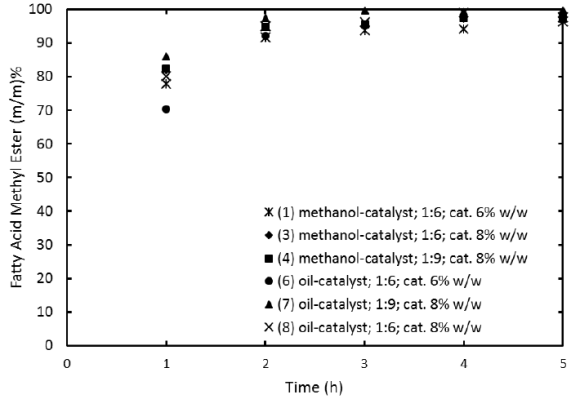
Fig. 1 Effect of reagents order addition on Fatty-Acid Methyl Ester Content profile. Reaction conditions: T = 328 K, rpm = 1000, Wcatalyst= 6 and 8 % w/w.
By contrasting the produced profiles in experiments in Figure 2, it can be concluded that the effect of methanol-oil molar ratio has a minor effect on reaction rate at all reaction times. This was an unexpected result and can be attributed to the fact that the reactor was equipped with baffles that promote mixing of methanol with oil and the catalyst thus avoiding mass transfer limitation at these molar ratios. Thus, a kinetic modelling is plausible to be conducted. It is also worth noticing that when studying this variable, the amount of Ca and Mg in the produced biodiesel was determined to be 145 and 2.0 mg/L, respectively. This analysis was conducted by ICP spectrometry as mentioned in section 2.4. Significant differences in the aforementioned values were not found among experiments. This indicates that the amount of catalyst available for homogeneous catalysis, Ca and Mg ions in the reaction mixture, was similar at all experiments when studying the effect of methanol-oil molar ratio. It is worth pointing out that these measurements were conducted at the end of the reaction time so these values could follow a different evolution with time but at the end of reaction the amount of leached ions is rather similar.
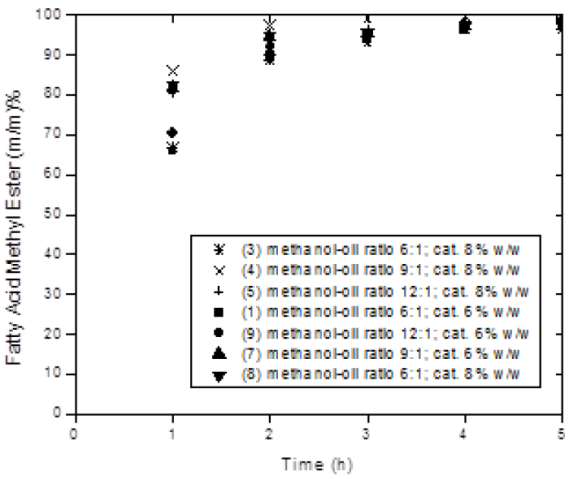
Fig. 2 Effect of methanol-oil molar ratio and catalyst loading on fatty acid methyl esters content. Reaction conditions: methanol-catalyst mixed at first (1, 9, 3, 4 and 5) and methanol-oil mixed at first (7 and 8), T = 328 K, rpm =1000, Wcatalyst= 6-8 % w/w.
Figure 3 shows evidence of the importance of catalyst loading. It can be observed that increasing the catalyst loading increases reaction rate and with the lowest catalyst loading of 2% presents a sigmoidal profile. This can be ascribed to the lack of active sites to readily transesterify the canola oil.
3.2 Kinetic modelling
The aforementioned experimental data were used to test the models proposed in
section 2.5 (Tables 2, 3, 4
and 5). Based on the estimated
parameters, confidence intervals and regression coefficients for all models, it
can be concluded that the model that best fits the experimental data is the one
with the Eley-Rideal mechanism and surface reaction as determining step with the
assumption of
Table 6 Estimated parameters and statistics.
| Parameter | Value | 95% confidence | Units |
|---|---|---|---|
| K1 | 19.013546 | 0.4281062 |
|
| K2 | 0.2187906 | 0.1902954 |
|
| K3 | 316.11977 | 7.1536297 |
|
| Statistics | |||
| R 2 | 0.9886145 | ||
|
|
0.9881498 | ||
| R msd | 0.0462578 | ||
| σ 2 | 0.1180812 | ||
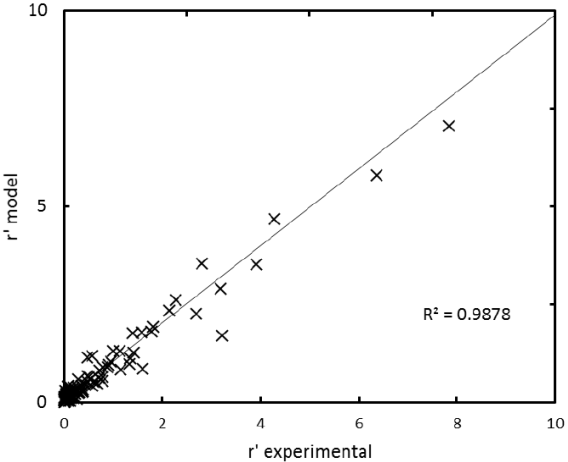
It is worth noticing that the adsorption equilibrium constant of methanol (K3) in the selected model, is several orders of magnitude greater than the surface reaction constants (K1 and K2), this is coherent with the assumption of the model, i.e the adsorption of methanol is faster than the surface reaction, which can be considered the controlling step of the reaction. Besides, it can be seen that the value of K2 is lower than K1, indicating that the reaction can be considered as irreversible. This is consistent with the use of an excess of methanol.
The r’ calculated with Equation 3 was plotted versus the r’ obtained from experimental data. This parity plot is shown in Figure 4.
The selected model is coherent with the mechanism reported by Peng-Lim et
al. (Peng-Lim Boey, Maniam, &
Hamid, 2011) using CaO (with other oil though) and also includes the
proposal of Kouzu et al. (Masato Kouzu, Hidaka,
Wakabayashi, & Tsunomori, 2010). According to the former,
methanol is dissociated upon adsorption onto the catalytic surface to mainly
form calcium methoxide, partial negative charge over the oxygen in this specie
attacks a carbonile group, as a consequence a stable diglyceride and one FAME
molecule is obtained and CaO is recovered. This may also be occurring over the
existent MgO. The same cycle will follow but now the diglyceride will be
transformed to monoglyceride, and finally the monoglyceride will be transformed
into glycerol and three molecules of FAME’s will be obtained. In addition and
according to the validated model, once glycerol is formed some will desorb and
some other will remain attached to the surface as glyceroxide (Sharma, Singh, & Korstad, 2011) and
this specie can also promote the methoxide formation. Then, the active site that
is occupied by this specie can still promote the reaction and thus reaction rate
is not really altered. Thus, instead of adding this step, desorption can still
be assumed to be fast enough compared to the other steps (this means it cannot
be the rate determining elementary step) and adsorption of glycerol to the
active site is assumed to be relatively weak
4. Conclusions
Biodiesel fulfilling the norm EN 14103 in terms of methyl esters content was produced from the transesterification of canola oil catalyzed by quicklime. This is a relatively low cost catalyst that can be successfully applied in the transesterification of vegetable oils. It was also concluded that none of the assessed methanol-oil molar ratio (6:1, 9:1 or 12:1) exerted a significant effect on reaction rate or final FAME content.
Regarding the kinetic modelling, it can be concluded that Langmuir-Hinshelwood-Hougen-Watson approaches are not adequate to reproduce FAME concentration profiles generated during the quicklime catalyzed canola oil transesterification.
A kinetic model based on Eley-Rideal mechanism with surface reaction as limiting step and weak adsorption of glycerol, for the transesterification of canola oil and methanol in the presence of quicklime as catalyst was concluded to best fit experimental results. The established equation can be applied to predict the methyl esters content with time and for process design purposes.
It can also be concluded that during the assessed process, triglycerides are not adsorbed onto the catalyst surface and due to the rapid adsorption of methanol the order of addition of reactants does not affect the reaction rate with this catalyst.











 nova página do texto(beta)
nova página do texto(beta)