1. Introduction
Because of exponential growing population, food demand is increasing, which in turn considerably increases agroindustrial wastes, becoming a relevant environmental problem. In addition to this, a greater coverage of services and products is also required, which leads to a search for alternatives and new sources of energy and raw materials.
Citrus fruit crops are the most abundant in the world (-120 million tons), where orange is the main product (Luengo, Álvarez, & Raso, 2013). Mexico is currently a leader in citrus production, being the fifth largest producer worldwide (4.6% of the total). Citriculture is an important social and economic activity, which is carried out on 23 states, of which the most important are Veracruz, San Luis Potosí, Tamaulipas, Puebla and Nuevo León (Bridgwater, 2006; SAGARPA, Secretary of Agriculture, Livestock, Rural development, n.d.).
Peels are the major solid waste of orange processing and represent about half of the fruit weight (Kantar et al.. 2018; Li, Smith, & Hossain, 2006; Lopez-Velazquez, Santes, Balmaseda, & Torres-Garcia, 2013a; Maran, Sivakumar, Thirugnanasambandham, &· Sridhar, 2013). As a consequence, it is one of the main wastes of the juice industry. These residues, without a well-defined application, may result in environmental disasters due to improper disposal (Marshall &· Farahbakhsh, 2013).
Biomass has been considered as a viable energy source and also as an alternative for the generation of raw materials produced from oil nowadays. Citrus agroindustrial residues are abundant and inexpensive material which can be used as biomass and therefore, as a sustainable alternative to solve an environmental and energy problem. Orange peels can be used as biomass for the production of high value-added products and fuels (Bridgwater & Peacocke, 2000; Ranzi et al., 2008). One of the most abundant agroindustrial wastes in Nuevo León state is sweet orange (Citrus sinensis) peel (Suárez-Jacobo et al., 2017).
Biomass conversion can be achieved by biological and thermochemical processes; the main difference between biological and thermal conversions is that biological conversion is a slow process with single or specific products such as ethanol or biogas, while thermal conversion takes place in short times and gives a variety of products (with potential to fuels or high-valued chemicals) and usually a catalyst is employed to upgrade the quality of the products (Bridgwater & Peacocke, 2000).
The main thermochemical processes used for biomass conversion are direct combustion, gasification and pyrolysis. Gasification and pyrolysis allow a greater valorization of the biomass and usually have high conversion efficiencies, potentially competitive costs, and considerable flexibility in scale of operation and range of products (Bridgwater & Peacocke, 2000). Furthermore, pyrolysis is the most versatile process due to the fact that solid (bio-char), liquid (bio-oil) and gaseous products are obtained and its application has the potential to diversify the energy-supply leading to a more secure and sustainable global energy-supply chain (Lopez-Velazquez et al.. 2013a). The quality of pyrolysis bio-oil from lignocellulose material as orange peel is too poor for direct use as fuel or fuel precursor (Babich et al., 2011; Lazdovica, Liepina, & Kampars, 2015). This bio-oil is usually characterized by its high viscosity, acidity, instability and low energy density, generally due to the presence of highly oxygenated compounds (Aho et al., 2008; Babich et al., 2011; Kelkar et al., 2014; Lazdovica et al., 2015; D.Wang, Xiao, Zhang. & He, 2010). Consequently, an upgrading process is necessary for the bio-oil before being used as a regular fuel, mainly involving the removal of oxygen in the bio-oils in order to decrease its acidity, which leads to an energy density increase (Demiral &· Sensöz, 2008; Lazdovica et al.. 2015; Pütün, Uzun, & Pütün, 2006; Wang et al., 2010), Catalytic pyrolysis has been suggested as an alternative to the direct conversion of biomass into high quality liquid bio-fuels (Kim et al., 2017; Lazdovica et al., 2015; Lazdovica, Liepina, & Kampars, 2016).
One of the methods used for bio-oil upgrading (usually promoting deoxygenation) is catalytic cracking (with zeolites, aluminosilicates and molecular sieves) (Bridgwater, 1996; Vitolo, Seggiani, Frediani, Ambrosini, & Politi, 1999), with the economic advantage that H2 is not required for hydrotreating (Huber & Corma, n.d.).The use of a suitable catalyst might change the reaction system during the pyrolysis process and result in an in-situ upgrading of the bio-oil. Heterogeneous catalysts are promising and adequate for use at pyrolysis conditions (450-550 °C) (Babich et al., 2011; Helwani, Othman, Aziz. Fernando, &· Kim, 2009; Kelkar et al., 2014; Kersten, van Swaaij, Lefferts, &· Seshan, 2007). The most studied catalysts in catalytic pyrolysis of biomass have been synthetic acid zeolites, mainly ZSM-5 (Aho et al., 2008: French & Czernik, 2010; Kelkar et al., 2014; Kim et al. 2017; Lazdovica et al., 2015, 2016; Li et al., 2014) and others such as MCM-41 (Adam et al., 2006; Antonakou. Lappas, Nilsen, Bouzga, & St0cker, 2006; Iliopoulou, E.V. Antonakou, Karakoulia, Vasalos, Lappas, 2007), MFI (Kelkar et al., 2014), Beta (Aho et al., 2007, 2008), Y (Aho et al., 2008; Nguyen, Zabeti, Lefferts, Brem, & Seshan. 2013) and mordenite (Aho et al., 2008) resulting in selectivity improvement by decreasing the content of oxygenated compounds, which promoted a better performance in the production of aliphatic and aromatic hydrocarbons (Aho et al., 2007; Atutxa, Aguado, Gayubo, Olazar, & Bilbao, 2005; Horne & Williams, 1996; Kelkar et al., 2014; Lappas, Samolada, Iatridis, Voutetakis, & Vasalos, 2002; Yaman, 2004) . Some authors (Kim et al.. 2017; Zhang et al., 2014) have previously proposed modification of the neutral SBA-15 as an alternative to zeolite use for biomass pyrolysis applications due to its higher thermal and hydrothermal stability . Furthermore, the use of low-cost materials such as dolomite and limestone has been reported, upgrading the pyrolysis oils to obtain higher liquid yields (Nokkosmäki, Krause, Leppämäki, & Kuoppala, 1998). Babich et al. (Babich et al., 2011) reported that Na2CO3 catalyst increases the heating value while decreasing the acidity of the bio-oil. CaO and dolomite have also been employed as tar cracking catalysts in thermal decomposition (Conesa & Domene, 2015; Wang et al., 2010), Wang et al. (Wang et al., 2010) tested CaO in the pyrolysis of catalytic corncob, resulting in effective deacidification, which in turn enhanced hydrocarbons production.
In this research, in-situ catalytic pyrolysis was studied, employing cheap, local minerals such as dolomite and clipnoptilolite as catalysts, being an attractive alternative to synthetic catalysts. In order to address not only a global environmental problem but also a local one, sweet orange (Citrus sinensis) peel (just the peel and not pulp) was chosen as biomass, being one of the most abundant agroindustrial wastes in Nuevo León state. The catalysts were tested for in-situ catalytic pyrolysis in their calcined and non-calcined form. Catalysts and the generated syngas and bio-oils were characterized.
2. Experimental
2.1 Biomass
Sweet orange (Citrus sinensis) peels were obtained from a local market. The biomass was dried in an oven at 60 °c for 24 h, prior to any further study. The dried biomass was grinded and sieved to a particle size between 250 and 2000 pm.
2.2 Catalysts
Dolomite and clinoptilolite were provided by local businesses (Desarrollo Ambiental Máximo and Grupo Filtrantes). Quartz sand (Sigma-Aldrich) was used as non-catalytic material. The catalysts were all grinded and sieved to a particle size between 250 and 2000 pm, and stored at 60 °C before use. To study the effect of catalyst calcination, these minerals were calcined at 800 °C for 4 hours. The calcination procedure consisted in heating at 800 °c for 4 h, and was performed using a microwave muffle furnace (CEM Phoenix). At the end, both non-calcined and calcined catalysts were collected. Therefore, four catalysts were obtained: non-calcined dolomite (NC-Dolomite), calcined dolomite (C-Dolomite), non-calcined clinoptilolite (NC-Clinoptilolite) and calcined clinoptilolite (C-Clinoptilolite).
2.3 Characterization of biomass and catalysts
2.3.1 Characterization of biomass and biomass-catalyst mixtures
To prepare the biomass-catalyst mixtures, catalysts were mecanically mixed with the dried biomasses and sieved to a particle size of < 2 mm. The thermochemical behavior of the mixtures of biomass with quartz sand (non-catalytic test) and the different catalysts was evaluated using thermogravimetric (TG) analysis coupled with differential scanning calorimetry (TGA-DSC, Linseis TGA-PT1000 model). In all cases, the ratio of biomass to catalyst used for the mixtures was 1:1. The conditions used for the thermogravimetric studies were heating from 25 to 750 °c (10°c/min ramp), with a nitrogen flow of 100 mL/min, 20 mg samples, with a particle size between 250 and 2000 pm.
2.3.2 Characterization of catalysts
The nature of functional groups in the catalysts was analyzed with Fourier-transform infrared spectroscopy (FTIR), using a Perkin Elmer Frontier instrument. Samples were analyzed using an attenuated total reflectance (ATR) accessory. The software used for spectra management was Spectrum (Perkin Elmer), in transmittance mode. All spectra were acquired with 64 scans and using a resolution of 4 cm-1. The wavenumber range of the tests was from 4000 to 350 cm-1.
In order to evaluate the presence of crystalline phases, the calcined and non-calcined catalysts were characterized by X-ray diffraction (XRD) using a Siemens model D-5000 diffractometer. The measurements were performed using Cu Ka radiation in the 2Θ range of 5-80°, with a 0.020° step every 4 seconds, at 25 °c. The pH of the catalysts was recorded according to the methodology described by Gurevich et al (Messina, Bonelli, & Cukierman, 2017), with a pH-meter (Orion 3 Star). Scanning electron microscopy (SEM), Zeiss EVO MA25 microscope, coupled with a Bruker XFlash 6110 energy dispersive X-ray spectrometer (EDX) was used to elucidate the morphology and the composition of the different catalysts.
2.4 In-situ catalytic pyrolysis test
The reactor setup used for the in-situ catalytic pyrolysis experiments consisted in a bench-scale stainless-steel semi-continuous reactor, where a biomass-catalyst mixture sample was placed in the bottom and heated from room temperature to a maximum of 600 °c, at an approximate heating rate of 10 °c/min. The generated biogas flowed through the system on its own. At the immediate exit of the pyrolysis reactor, a heated stainless-steel tube was placed, which was maintained at 70 °c during the experiments, in order to avoid early condensation of the bio-oils. After the heated tube, a Biichner flask was present, which was cooled at a constant temperature of 3 °c with ice in order to properly condensate the generated bio-oil. The non-condensable compounds continued flowing, first through two glass tubes (impactors) which were also cooled at 3 °c and acted as a trap for organic products which could have exited the Biichner flask without condensing. After the impactors, the gas continued flowing through a sampling port and finally the vent. A total sample mass of 200 g (biomass:catalyst ratio of 1:1) was used in all tests. The condensed fractions were collected in the aforementioned Büchner vacuum flask, and then transferred to individual glass bottles and stored at 3 0C in absence of light for further analysis. Biogas was obtained from the sampling port and stored in Tedlar bags at three different temperature ranges: 200 ֊ 290 °c (15 min, Temperature 1), 300 ֊ 340 0C (5 min, Temperature 2), and 380 ֊ 490 °c (15 min, Temperature 3), according to the data obtained by the TG tests.
2.5 characterization of the pyrolysis products
Gas chromatography coupled to a thermal conductivity detector (GC-TCD) was used in order to evaluate the content of primary gases such as H2, CO, CO2 and CH4 in the syngas. The instrument used was a Hewlett Packard 5890 Series II, which operated using a custom column with the characteristics: 15' 1/8" SS column, Carboxen 1000 resin, 60/80.
In order to separate the organic phase (bio-oil) from the condensate fraction, a liquid-liquid extraction was performed using dichloromethane (Tedia, HPLC grade) as solvent. The bio-oil:solvent ratio used was 1:2 (volume). The extracted organic phase was evaluated using gas chromatography coupled to mass spectrometry (GC-MS), with an Agilent 6890 series gas chromatograph, coupled to a MS 5973 Network mass selective detector. An HP5-MS (Agilent) column was used, and the m/z range was 30-550. Helium (99.997%) was used as eluent gas for the GC-TCD and GC-MS tests.
3. Results and discussion
3.1 FTIR Spectroscopy
Figure 1 shows the FT-IR spectra of the mineral catalysts before and after calcination. For NC-Dolomite and NC-Clinoptilolite, characteristic bands are observed for stretching vibrations in the range of 3000-3600 cm"1 (Figure 1a,c) (Ji, Ge, Balsam, Damuth, & Chen, 2009; Korkuna et al., 2006; Lavat, Grasselli, & Lovecchio, 2015) and for bending vibrations at 1640 cm-1 (Figure 1a) for NC-Dolomite and 1625 cm"1 for NC-Clinoptilolite (Figure 1a) (Ji et al., 2009; Lavat et al., 2015); this due to adsorbed surface water, for both catalysts. As expected, after calcination, water is eliminated from the surface of the minerals; for C-Dolomite and C-Clinoptilolite the aforementioned signals disappear (Figure 1b, d).
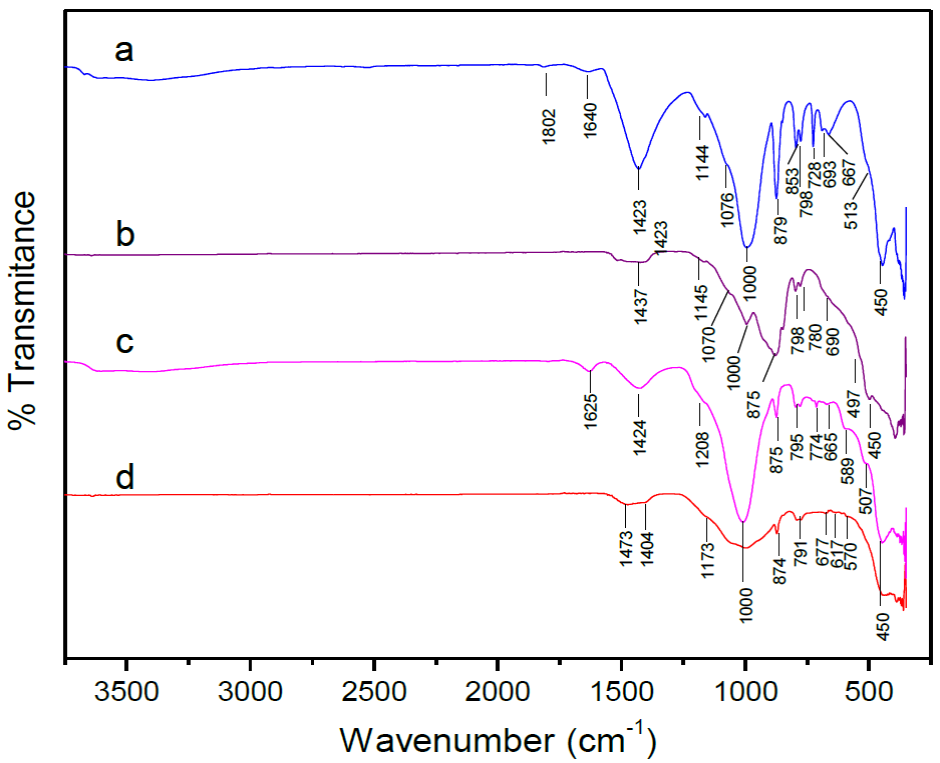
Fig. 1 FTIR spectra of purc mineral catalysts a. NC-Dolomitc, b. C-Dolomite, C NC-Clinoptilolite and d. C-Clinoptilolitc
In Figure 1a,b, the signals at 1423 and 1437 cm1 are associated with assymetric C-O vibrations from the carbonate group (Correia et al., 2015; Lavat et al., 2015: Sasaki, Yoshida, Ahmmad, Fukumoto, & Hirajima, 2013): in the same case, the bands at 879 and 875 Cm1 are related to bending vibrations of the СаСОз groups in dolomite (Lavat et al., 2015). For NC-Dolomite (Figure 1a), a band is observed at 853 cm1, which is attributed to bending vibrations of both the CO3 2" group outside the plane (Correia et al., 2015) and the Mg-C bond (Jarrahian. Seiedi, Sheykhan, Sefti, & Ayatollahi, 2012; Sasaki et al. 2013) present in dolomite. On the other hand, the signal at 728 cm"1 is related to bending vibrations from the CO3 2" functional group inside the plane (Correia et al., 2015; Ji et al., 2009) and the Ca-C bond (Jarrahian et al., 2012; Sasaki et al., 2013). These signals are absent in the C-Dolomite spectrum, or they may be present with a significantly decreased intensity, since after calcination at 800 °c, the carbonate groups in dolomite (CaMg(CO3)2) are eliminated, leading to formation of the CaO and MgO oxides (Sasaki et al., 2013).
The bands in the range of 1400-1500 Cnr1 are attributed to stretching vibrations of the C-O bond in the CO3 2- group for NC-Dolomite; when dolomite is heated above 700 °c, these signals are associated with СаСОз and the CO2 formed by dolomite decomposition (Ji et al., 2009: Sasaki et al., 2013). The produced CO2 is initially adsorbed in the CaO and/or MgO structures. However, above 800 °c, complete carbonate decomposition has been reported, and CO2 is no longer adsorbed in the calcium and magnesium oxides (Sasaki et al., 2013). The bands at 1444 and 1145 cm"1 are attributed to the S1O4 present (Lavat et al., 2015). A significant decrease in signal intensity is clearly observed for C-Dolomite, while other bands completely disappear in comparison with NC-Dolomite; this is a consequence of structural transformations and decarbonation.
Since clinoptilolite is mainly composed by S1O2 and Al2O3, the bands presented in this catalyst (before and after calcination) are related mainly to vibrations of the Si-O and Al-O bonds. The signals at 1000 cm-1 are associated to these event, and its position is related with the Si/Al ratio (Favvas et al., 2016). In the clinoptilolite spectra (Figure 1c,d) no significant change was observed related to a change in the band position for NC-Clinoptilolite and C-Clinoptilolite, therefore, no evidence is present of a change in the Si/Al ratio, which was later confirmed in the EDX results.
In Figure 1c,d, bands present in the 1200-400 cm1 range are associated with Si-O(Si) and Si-O(Al) bonds vibrations (Korkuna et al., 2006), while the signals in the range of 500-700 cm1 are attributed to pseudo-lattice vibrations. Bands below the 400 cm1 line are related with lattice vibrations [43]. Finally, the signals at 420-500 cm1 represent bending vibrations from the Si-O and Al-O bonds (Perraki & Orfanoudaki, 2004).
3.1 XRD
Crystalline phases present in calcined and non-calcined natural mineral catalysts were detected by XRD. Figure 2a shows the diffraction pattern for NC-Dolomite, where the main dolomite MgCa(CO3)2 phase is observed (Charusiri & Vitidsant, 2017; Elbaba & Williams, 2013; Paulo & Dittrich, 2013; Xie et al., 2016). The presence of other phases such as aragonite and calcite (СаСОз phases), MgCO3, CaO, halite (NaCl) and quartz were also observed (Paulo & Dittrich, 2013). For C-Dolomite, disappearance of the carbonate phases is observed (except calcite, the most stable phase), with the appearance of high-magnesium calcite, CaO and MgO phases (Charusiri & Vitidsant, 2017; Elbaba & Williams, 2013); quartz signals remain present after calcination. The observed phase changes occur due to transformation of dolomite to Ca and Mg oxides, promoted by the calcination treatment.
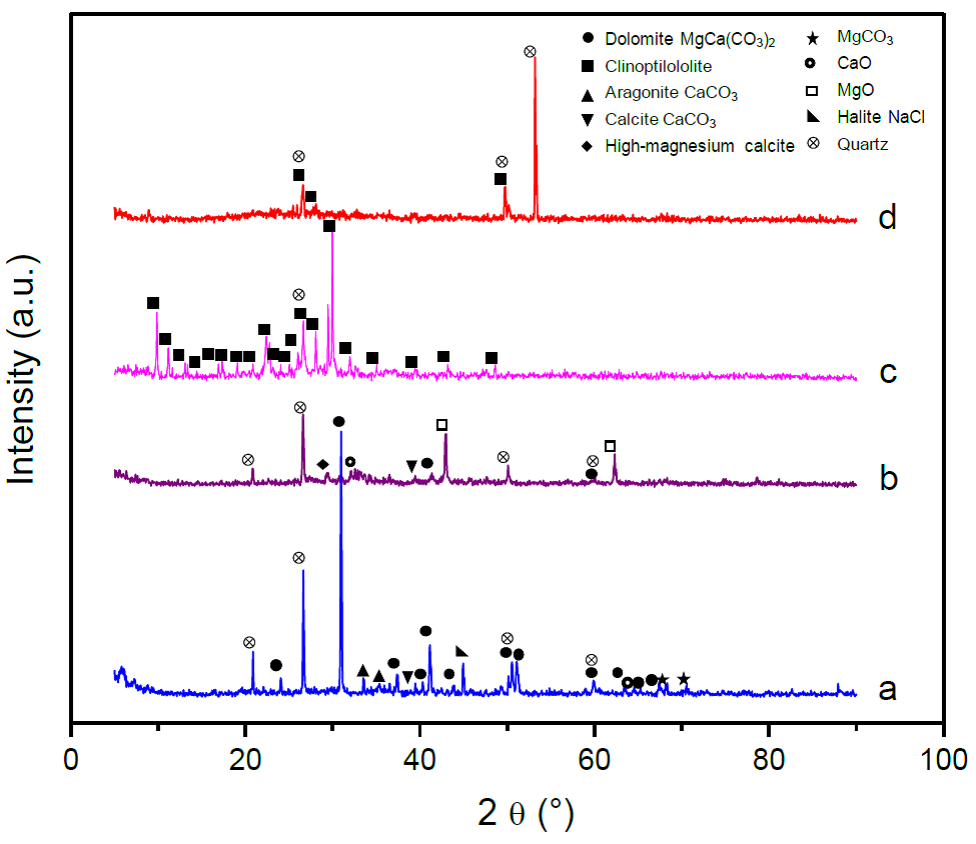
Fig. 2 XRD patterns of mineral catalysts a. NC-Dolomitc, b. C-Dolomite, C NC-Clinoptilolite and d. C-Clinoptilolite.
Clinoptilolite is mainly formed by aluminosilicates, where the microstructure comprehends a tetrahedron tridimensional lattice formed by SiO4 and AlO4, with silicon and aluminum atoms at the center and oxygen atoms at the corners (Faghihian, Talebi, & Pirouzi, 2008); other elements may be present, since AlO4 excess promotes local negative charges which are neutralized with cations such as Na+, K+, Ca2+, Sr2+, Mg2+, etc., via electrostatic forces (De J. Montes Luna et al., 2018; Olguin Gutiérrez. 2007). The obtained diffraction pattern for NC-Clinoptilolite is in agreement with previous reports by other authors for natural zeolites of the clinoptilolite type (Adriano Macas, 2012; Favvas et al., 2016; Korkuna et al., 2006; Rodríguez-Méndez et al., 2014); it also presents quartz as impurity (common in natural minerals), which remains in the diffraction pattern after calcination. However, characteristic natural zeolite diffraction peaks mostly disappear after the thermal treatment, this may be due to the collapse of the zeolite structure, loss of crystallinity and formation of amorphous phases (J.-C. Kim et al., 2014). Quartz may form as product of temperature increase; the complete zeolite structure decomposition temperature is 900 °c, which is the reason why some clinoptilolite phase peaks are still present in the diffraction pattern of C-Clinoptilolite (Guvenir, 2005).
3.2 SEM
The morphology of the mineral natural catalysts, both in their calcined and non-calcined form, was evaluated. Figure 3 shows the SEM micrographs, where dolomite shows rounded particles and a heterogeneous particle size distribution (Correia et al., 2015; Sasaki et al., 2013); small aggregates are observed over the bigger-size particles. In the C-Dolomite micrograph (Figure 3b), a higher amount of aggregates and smaller particles are observed, in comparison with the image for NC-Dolomite (Figure 3a), which is in agreement with previous works, which report a decrease in particle size when dolomite is calcined above 600 °c (Sasaki et al., 2013). For NC-Dolomite, smooth particles are observed, while after calcination roughness increases, along with the pores and smaller aggregates amount (over the bigger particles), which again agrees with previous reports (Correia et al., 2015; Lavat et al., 2015; Sasaki et al., 2013). This may be observed due to an increase in surface area and porosity after calcination due to decomposition of Ca and Mg carbonates, which promote pore forming after CO? desorption from CaO and MgO sites. Therefore, calcination enhances the structural properties of dolomite, which may increase the catalytic properties of this material.

Fig. 3 SEM micrographs of natural minerals catalysts a. NC-Dolomitc, b. C-Dolomitc, C NC-Clinoptilolite and d. C-Clinoptilolitc. EHT: 20kV, Mag: 2.00k.
Non-calcined clinoptilolite (Figure 3c) presented a morphology which consisted in plates and ribbons, as reported for natural clinoptilolite present in sedimentary rocks. Rounded particles are also observed, and the particle size distribution appears to be similar to that of dolomite (Guvenir, 2005). C-Clinoptilolite presents a morphology similar to that of NC-Clinoptilolite, with a clear decrease in the presence of ribbon-shaped particles over bigger particles, which in turn show a smoother surface. These may be related to thermal decomposition, where the zeolitic structure partially collapses, since the temperature at which the structure is completely destroyed is 900 0C (Guvenir, 2005; Sasaki et al., 2013). This was confirmed in the diffraction patterns previously discussed in Section 3.2.
3.3 EDX
Figure 4 shows the energy dispersive X-ray spectra for the calcined (Figure 4b,d) and non-calcined (Figure 4a,c) catalysts, along with the elemental surface compositions. The chemical composition of dolomite is CaMg(CO3)2; however, other elements are present as impurities due to the natural origin of the mineral. The EDX analysis showed that for C-Dolomite, oxygen content decreases, while Mg and Ca distributions increase. This is directly related with the decarbonation process, where CO2 is released by thermal decomposition of the Mg and Ca carbonates (Algoufi, Kabir, & Hameed, 2017). This confirms the previous evidence found in the previously discussed results of FTIR and XRD.
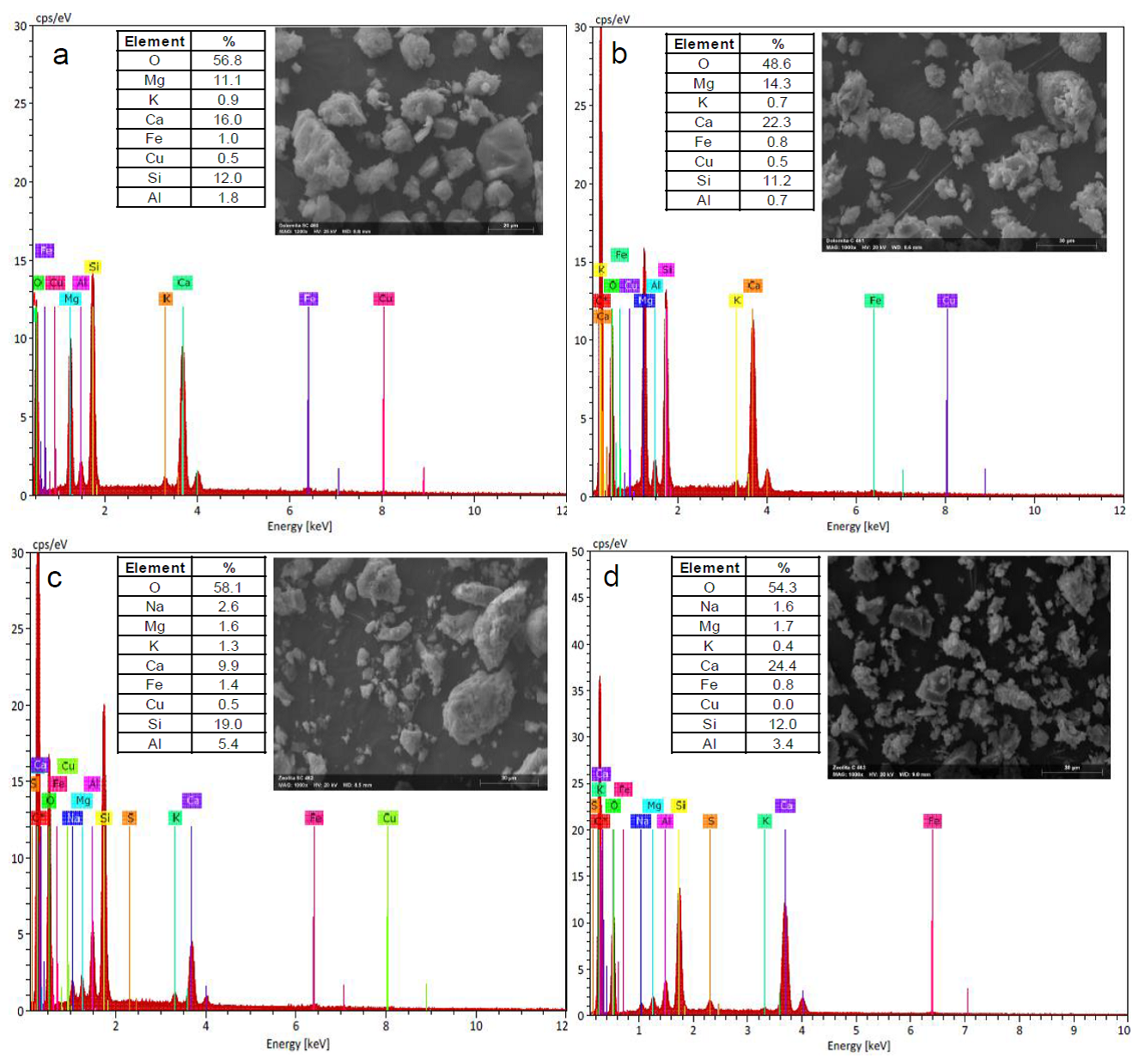
Fig. 4 EDX analysis of natural mineral catalysts a. NC-Dolomite, b. C-Dolomite, C. NC-Clinoptilolite and d. C-Clinoptilolito.
Clinoptilolite is mainly composed by aluminosilicates; however, some cations such as Na+, K+, Ca2+, Sr2+ and Mg2+ may also be present (De J. Montes Luna et al., 2018). The EDX analysis shows a higher Ca content for NC-Clinoptilolite compared with previous reports (Favvas et al., 2016; Messina et al., 2017); this causes a reduction in the Si and Al contents, probably due to the presence of СаСОз and CaO. In the case of C-Clinoptilolite, an increase in Ca content is observed, with a decrease in the content of Si, Al and O, probably due to desilication and/or dealumination processes. This events may be the results of calcination and partial destruction of the crystalline zeolitic structure (Wang, Yokoi, Namba, & Tatsumi, 2016).
3.4 Thermogravimetric analysis
3.4.1 Thermal behavior
Figure 5 shows the thermogravimetric analysis for the non-catalytic and catalytic biomass mixtures. The dTG curves (Figure 5b) clearly show the presence of three main decomposition events, their temperature ranges were identified as: 200-290 °c, 300-340 0C and 380-490 0C According to these results, the same temperature sampling ranges were used in the catalytic in-situ pyrolysis tests performed in the semi-continuous reactor.
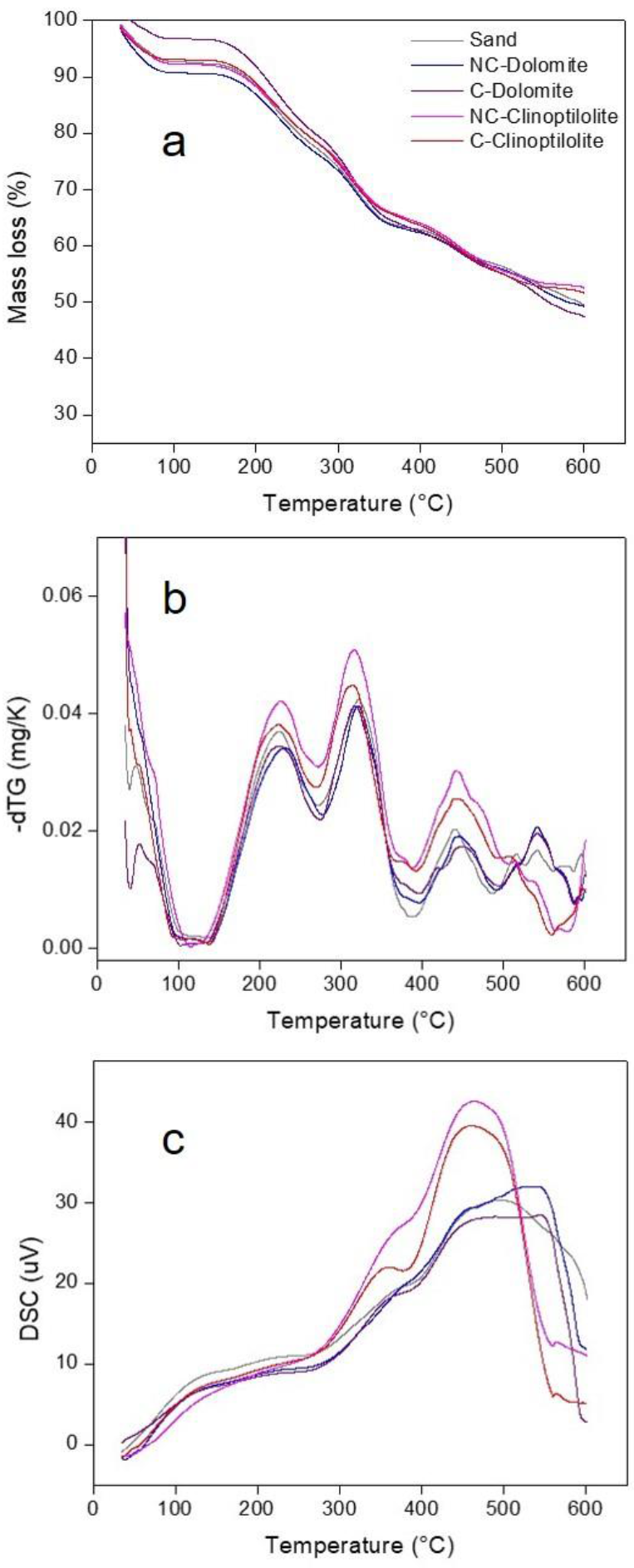
Fig. 5 Thermogravimetric analysis of orange peel mixtures with catalysts: a. TG, b. dTG and C DSG (Condi-tions: 20mg of sample; ramp: 10"C/min; flow rate: 100 mL N2/min).
Figure 5a shows the TG curves, where the mass decrease below 120 °c is associated with physisorbed water desorption, and possibly water-soluble species that volatilize at low temperatures (Lopez-Velazquez et al., 2013a; Miranda, Bustos-Martinez, Blanco, Villarreal, & Cantú, 2009a; Santos, Dweck, Viotto, Rosa, & de Morais, 2015). Two overlapping steps (corresponding to a mass loss of approximately 20%) are observed in the range of 160-300 °c, which may be attributed to initial hemicellulose and partial cellulose decomposition (Lopez-Velazquez et al., 2013a; Miranda et al., 2009a). At the temperature range of 300-600 °c, at least two slope changes are observed, which correspond to overlapping mass-loss stages associated with completion of hemicellulose decomposition, ongoing cellulose decom position and lignin degradation (Lopez-Velazquez et al.. 2013a; Santos et al., 2015); this stage shows a mass loss between 25-30%. After 600 °c, complete degradation of the orange peels is expected, according to previous reports (Aguiar, Márquez-Montesinos, Gonzalo, Sánchez, & Arauzo, 2008; Kim et al., 2017).
According to literature, dolomite may show mass losses in the 600-850 0C range, due to the decarbonation process (Djayaprabha, Chang, Shih, & Chen, 2017), which is the process attributed to the additional mass loss event at the end of the TG curve for NC-Dolomite. For clinoptilolite, physisorbed water loss is observed until 200°c, with continuous reticular water loss in the 200-700 0C range, which is characteristic of this type of natural zeolite in comparison with other more stable zeolites such as mordenite. This events may add to the mass loss of the biomass-catalyst mixtures (Ates & Hardacre, 2012).
3.4.2 Kinetic analysis
Model-free isoconversional methods have been reported in literature as useful for activation energy (Ea) calculations in thermally activated reactions (Starink. 2003). Therefore, two of these methods were used in this work, namely the Flynn-Wall-Ozawa (FWO) (Jain. Mehra, & Ranade, 2016; Venkatesh, Ravi, & Tewari. 2013) and Kissinger-Akahira-Sunose (KAS) (Jain et al.. 2016; Lopez-Velazquez, Santes, Balmaseda, & Torres-Garcia, 2013b) methods. The Kissinger equation was also used to calculate the pre-exponential (A) factor, and the kinetic constant (k) was also obtained with the expression for Arrhenius Law (Jain et al., 2016; Urych, 2014). The final equations for the methods are resumed in Table 1, where β represents the heating rate in °c/min, α represents the conversion degree, T is the temperature in K and Tmax is the temperature associated with the maximum intensity of the events (peaks) observed in the DTG curves.
Table 1 Equations of isoconvertional methods used in this work
| Method | Equation |
|---|---|
| FW() |
|
| KAS |
|
| Kissinger |
|
For the calculation of activation energies, TGA tests were performed at different heating rates (β): 5, 10 and 15 °c/min. In order to clearly evaluate the effect of the heating rate in the thermal events of orange peel pyrolysis, Figure 6 shows the DTG curves obtained for all three β values used. At least four important observations can be made from this plot: first, all DTG curves showed the same main behavior, with three main events clearly present in all cases, which may suggest the thermal behavior is independent of the heating rates. However, the intensity of the DTG peaks shows an inverse relation compared to the heating rate, with the maximum mg/K value decreasing with increasing β, which means the slope associated with mass loss becomes less pronounced. The sensibility of the DTG analysis also decreases with increasing heating rates, with small peaks overlapping with the main DTG events when β = 5 °c/min; however, at 10 °c/min, only the main peaks can be observed. At the final heating rate, even the two main events are overlapped with themselves. Finally, the logical shifting of the DTG peaks to higher temperatures with increasing β is also observed.
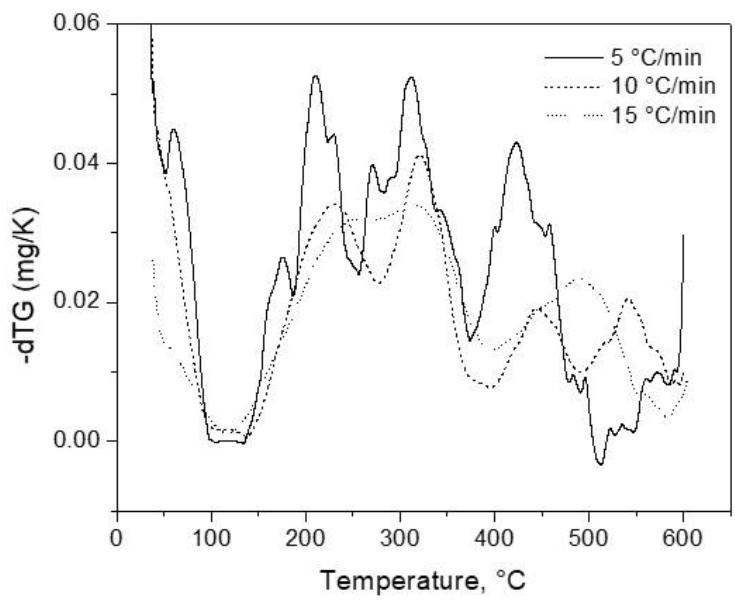
Fig. 6 DTG curves for the catalytic mixture of orange peel with NC-Dolomite at different heating rates.
Figure 7 shows the results of the apparent activation energy calculations using the FWO and KAS methods previously described, for conversion degrees between 0.1 and 0.9. Almost identical results were obtained with the FWO and KAS equations, showing these two isoconversional methods closely describe the pyrolysis reactions performed in this work. Three maximum Ea peaks were observed at 0.3, 0.6 and 0.8 conversion values, which are associated with the three main decomposition events previously identified in the DTG curves. It is very evident from these results that the non-catalytic test showed the highest Ea values of all the mixtures. López-Velazquez et al. (Lopez-Velazquez et al., 2013b) reported apparent activation energies between 200 and 250 kJ/mol for pure orange peel samples, at similar α values. Since the Ea calculated for the non-catalytic test in this work varies between 600 and 1900 kJ/mol, this suggests the inert material in the mixtures negatively affect the pyrolysis process, blocking the biomass decomposition with quartz sand absorbing most of the termal energy to increase its temperature, therefore requiring higher energies for the orange peel to decompose. On the other hand, the catalytic mixtures showed a drastic decrease in the Ea values, with maximums of 320 and 300 kJ/mol for conversion degrees of 0.6 and 0.8, respectively, observed in the mixture with NC-Zeolite. However, catalyst calcination promoted an even more marked apparent activation energy decrease, with values in the range of 130 and 145 kJ/mol for α = 0.6, and between 85 and 95 kJ/mol for α = 0.8, when C-Dolomite and C-Zeolite were present in the mixtures. These values are even lower than those reported by López-Velázquez et al., which shows the calcinated catalysts have a positive effect on the Ea, which makes it more attractive to use them in mixtures with orange peels for pyrolysis applications than using the pure biomass.
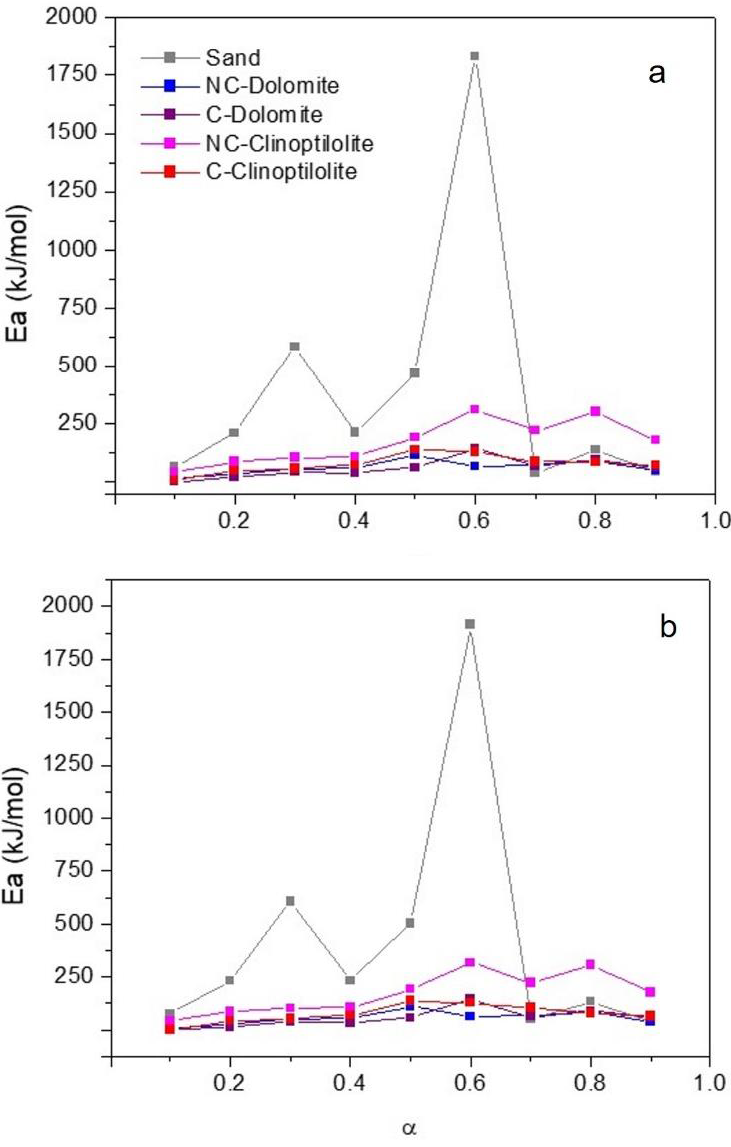
Fig. 7 Variation of the apparent activation energy Ea with the conversion degree, for different non-catalytic and catalytic mixtures of natural minerals with orange peels. Calculation performed by using: a. the FWO method, b. the KAS method.
The pre-exponential factor (A) was evaluated with the intersection value
obtained from the regression equation calculated when plotting
Table 2 shows the kinetic parameters obtained, where peaks 1, 2 and 3 correspond to the main mass loss events observed previously in the DTG curves.
Table 2 Pre-exponential factors and kinetic constants values calculated with the method of Kissinger
| Peak 1 | Peak 2 | Peak 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | A1 (min-1) | k 1 (min-1) | R 2 | A2 (min-1) | k 2 (min-1) | R 2 | A3 (min-1) | K 3 (min-1) | R 2 |
| Sand | 3.09E+04 | 0.2192 | 0.9899 | 6.89E+21 | 0.7818 | 0.8576 | 9.33E+13 | 0.4214 | 0.8122 |
| NC-Dolomite | 2.99E+04 | 0.2167 | 0.9900 | 6.89E+21 | 0.7818 | 0.8576 | 4.17E+03 | 0.1319 | 0.8833 |
| C-Dolomite | 5.79E+04 | 0.2296 | 0.9903 | 2.99E+38 | 1.3629 | 0.9906 | 6.10E+13 | 0.4128 | 0.9951 |
| NC-Clinoptilolite | 1.05E+04 | 0.2000 | 0.9949 | 1.55E+27 | 0.9721 | 0.9777 | 6.14E+04 | 0.1628 | 0.8391 |
| C- Clinoptilolite | 1.38E+04 | 0.2045 | 0.9894 | 1.55E+27 | 0.9721 | 0.9777 | 7.66E+08 | 0.2779 | 0.9090 |
The kinetic constants suggest a modest effect of the catalysts on the reaction rate of orange peel pyrolysis.
However, a consistent behavior is also observed, with & values increasing with calcination of the catalysts (except for Clinoptilolite in peak 2). C-Dolomite showed the highest & values of all mixtures for the first two mass loss events.
To the best of our knowledge, this is the first time apparent activation energies are reported for catalytic pyrolysis tests, since most of the previous works have only worked with pure biomass samples or simple carbohydrates.
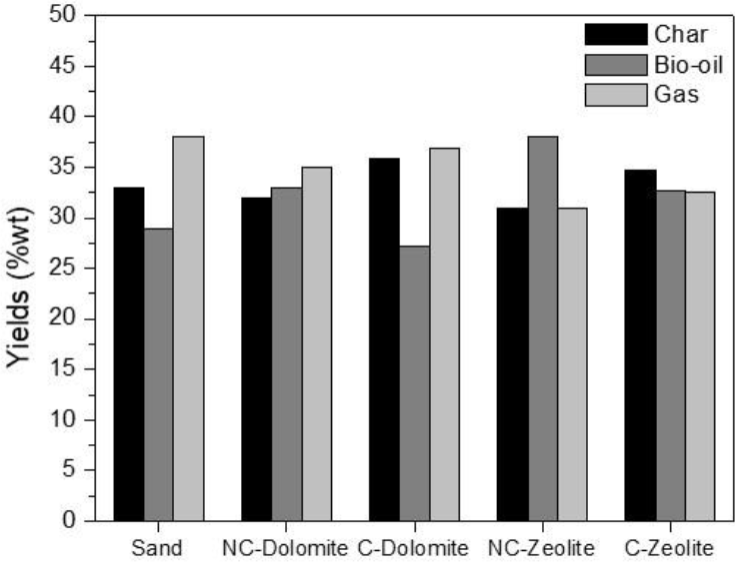
3.5 Yields of in-situ catalytic pyrolysis
Figure 8 shows product yields for the in-situ catalytic pyrolysis of orange peels, where the performance of different catalysts is compared with the non-catalytic test (quartz sand), where the values were calculated by mass balance. It is observed that calcination of the catalysts promotes an increase in syngas yield (5.4 and 5.2% increase for dolomite and clinoptilolite, respectively), likely due to bio-oil cracking deoxygenation (Bridgwater, 1996; Corma, Huber, Sauvanaud, & O'Connor, 2007; Vitolo et al., 1999). On the other hand, non-calcined catalysts showed selectivity for higher bio-oil production, with yields 21.3 and 16.2% higher for dolomite and clinoptilolite, when compared with the calcined materials. An increase in char yield is observed for the calcined catalysts; this was also observed in the TG results, and may occur due to CO2 adsorption over the surface of CaO and MgO species formed after calcination (Sasaki et al.. 2013). Therefore, higher selectivity for gases different tan CO2 in the syngas may be expected. Though the yield increments may appear low, the main effect of the presence of catalysts in the mixtures was the product selectivity, which will be discussed in the next sections of this work.
Table 3 shows all organic compounds identified by GC-MS analysis of the bio-oils obtained from in-situ catalytic pyrolysis of orange peels, which are similar to results reported in previous works (Miranda, Bustos-Martinez, Blanco, Villarreal, & Cantú, 2009b). It was observed that for all cases, valuable chemical compounds (building blocks) were generated. Cyclotene (compound widely used in fragrance and cosmetics industry, with world consumption calculated at 0.1-1 ton/year (Scognamiglio, Jones, Letizia, & Api, 2012) and guaiacol (model bio-oil molecule), used for biofuel synthesis (Pereira et al., 2018; Zhao, Li, Bui, & Oyama, 2011)) were found to be the main organic compounds found in the bio-oils generated from the non-catalytic and the catalytic tests where non-calcined materials were used. In the case of the calcined catalysts, the main product was D-Limonene, which is also widely used in the food, fragrancy, cosmetics and cleaning products industries, and has had a recent interest in the pharmaceutics area (Vieira, Beserra. Souza, Totti, & Rozza, 2018).
Table 3 Organic compounds distribution in the bio-oils, obtained with GC-MS.
| % O | Compound | % Relative area | ||||
|---|---|---|---|---|---|---|
| Saud | NC Dolomite | C-Dolomite | NC-Clinoptilolite | C- Clinoptilolite | ||
| 38.1 | 2-Hydroxy-3-methylpyran-4-one | - | 7.1 | - | - | - |
| 38.1 | 5-Hydroxymethyfurfural | 4.9 | 6.3 | - | - | - |
| 31.5 | 2,4-Dihydroxyacetophenone | - | - | - | 4.0 | - |
| 29.1 | Catechol | 8.0 | 5.9 | - | 5.9 | 3.4 |
| 28.8 | Cyclotene | 17.4 | 12.3 | - | 15.3 | 8.1 |
| 25.8 | Guaiacol | 12.6 | 13.8 | 10.1 | 12.3 | 5.7 |
| 25.8 | 3-Methyl catechol | 2.6 | - | - | - | - |
| 25.4 | Ethyleyclopentonolone | 7.1 | - | 2.2 | - | 2.3 |
| 23.5 | Methyl benzoate | - | - | - | - | 4.1 |
| 21.3 | 2-Metoxi-4-vinilphenol | 2.1 | 3.9 | 3.0 | 1.7 | 2.0 |
| 21.0 | 4-Ethylguaiacol | 3.3 | 3.6 | 2.8 | - | - |
| 14.8 | o-Cresol | 5.3 | 5.0 | - | 8.3 | 2.1 |
| 14.8 | m- Cresol/ p-Cresol | 6.9 | 7.0 | 7.3 | 8.5 | 3.4 |
| 14.7 | 3-Methoxypyridine | 5.5 | - | - | - | 4.9 |
| 14.5 | 2,3-Dimethyl-2-cyclopenten-1-one | - | 4.9 | 5.1 | 5.6 | 2.1 |
| 13.1 | 2,4-Dimethylphenol (m-Xylenol) | 5.3 | 6.7 | 7.0 | 5.4 | - |
| 13.1 | 3.4- Dimethylphenol (o-Xylenol) | - | 5.3 | - | - | - |
| 13.1 | 4-Ethylphenol/ 3- Ethylphenol | 3.7 | - | 3.2 | - | - |
| 0.0 | Limonene | - | - | 25.4 | - | 53.9 |
| Not identified | 15.3 | 18.2 | 33.9 | 32.9 | 7.7 | |
The non-catalytic test along with the pyrolysis tests performed with non-calcined catalysts showed a very prominent formation of highly oxygenated organic compounds (calculated by the mass percentage of oxygen in the molecule, and represented as %O in Table 2), with NC-Clinoptilolite having more than 39% of the relative area corresponding to organic compounds with more than 20% oxygen content, while NC-Dolomite and the non-catalytic test showed values of 53 and 58% for the same parameter, respectively. On the other hand, the percentage of relative area attributed to compounds with oxygen contents higher than 20% was only 18 and 25% for C-Dolomite and C-Clinoptilolite, respectively.
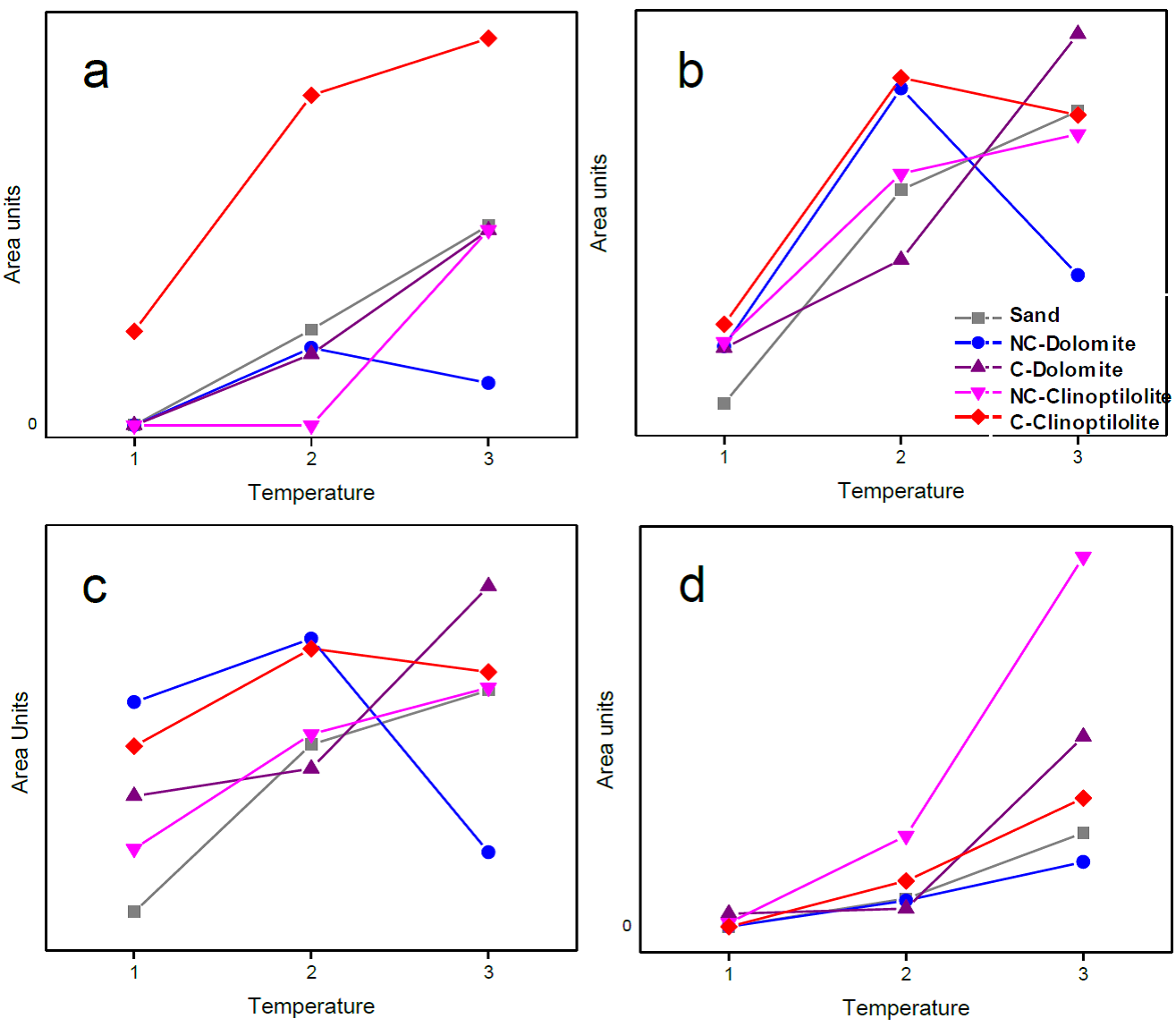
Therefore, calcination of the catalysts is observed to clearly promote formation of less-oxygenated compounds, which increased the quality of the generated bio-oils. Loss of oxygen in the bio-oil is also associated with reduced acidity; Table 4 shows the pKa of most of the identified products. In general, formation of less acidic compounds was favored by the calcined catalysts. Figure 9 shows the results obtained by GC-TCD for the primary gases distri bution (H2, CO, CH4, CO2) in the obtained syngas for the previously described tests. A significant difference in hydrogen production (Figure 9a) was observed, where C-Clinoptilolite showed the highest values for all temperatures. In this case, the non-catalytic test showed hydrogen content results similar to that of the rest of the catalysts (except C-Clinoptilolite), while NC-Dolomite showed the lowest H2 content in the syngas sampled at the highest pyrolysis temperature. A higher hydrogen production may be related with a higher calcium content present in C-Clinoptilolite, which was previously confirmed by EDX; previous works have reported pure CaO acts as pyrolysis catalyst, favoring water-gas shift reaction and methane reforming, with the consequent decrease in CO content in the syngas (Veses et al., 2014; Widyawati, Church, Florin, & Harris, 2011). A different behavior is therefore observed for CO content (Figure 7b), where while C-Dolomite shows the highest carbon monoxide values for the first two sampling temperatures, at the highest temperature CO production decreases, according to the previous discussion. Figure 9d shows all curves follow a similar trend, with CH4 content increasing with higher temperatures. In this case, NC-Clinoptilolite showed the highest methane content at the highest sampling temperatures.
Table 4 Acidity values (represented by pKa) of the organic compounds identified by GC-MS.
| Compound | pKa | Rcf. |
|---|---|---|
| 3-Methoxypyridine | 4.8 | (Guillory, 2010) |
| 2,4-Dihydroxyacetophenone | 7.9 | (Listing Compounds, n.d.) |
| 2-Hydroxy-3-methyl-2-cyclopenten-l-one (Cyclotene) | 9.3 | (Yeast Metabolome Database,n.d.) |
| 3-Ethyl-2-hydroxy-2-cyclopenten-l-one (Ethylcyclopentenolone) | 9.4 | (Listing Compounds, n.d.) |
| 2- Hydroxy- 3- methylpyran- 4- one | 9.5 | (Listing Compounds, n.d.) |
| Catechol | 9.5 | (Serjeant & Denipsey, 1979) |
| 3-Methyl catechol | 9.6 | (Listing Compounds, n.d.) |
| 4-Ethylphenol / 3-Ethylphenol | 9.9 | (Pearce & Simkins, 1968) |
| 2-Metoxi-4-vinilphenol | 10.0 | (Listing Compounds, n.d.) |
| Guaiacol | 10.0 | (Pearce & Simkins, 1968) |
| 4-Ethylguaiacol | 10.3 | (Listing Compounds, n.d.) |
| o-Cresol | 10.3 | (Shiu, Ma, Varhaníčková, & Mackay, 1994) |
| m-Cresol / p-Cresol | 10.3 | (Pearce & Simkins, 1968) |
| 3,4-Dimethylphenol (o-Xylenol) | 10.5 | (Listing Compounds, n.d.) |
| 2,4-Dimethylphenol (m-Xylenol) | 10.6 | Serjeant & Denipsey, 1979) |
| 5-Hydroxymethylfurfural | 13.7 | (Listing Compounds, n.d.) |
| 2,3-Dimethyl-2-cyclopent-1-one | --- | --- |
| Limonene | --- | --- |
| Methyl benzoate | --- | --- |
4. Conclusions
In this work, the effect of the use of natural minerals and the impact of calcination treatment on these catalysts was studied. Impurities were found by XRD and EDX, while typical dolomite and clinoptilolite functional groups were identified by FTIR. SEM micrographs showed changes in particle size and morphology of the catalysts after calcination, while EDX confirmed the Si/Al remained intact after thermal treatment of the natural minerals, although some degree of desilication and dealumination was observed, especially in the case of clinoptilolite.
The presence of minerals slightly altered the mass-loss behavior, as observed by the TG results. However, pyrolysis tests in the semi-continuous reactor showed similar solid yields for all tests, with the values of this parameter varying only between -6 and +9% for the catalytic tests, compared with the non-catalytic mixture, therefore showing a reduced effect of the minerals on the mass loss for higher-scale tests.
Natural minerals also significantly changed the kinetic parameters of the pyrolysis process, drastically decreasing the apparent activation energies of the catalytic mixtures compared with the quartz sand mixture. Calcination of the natural minerals promoted an even more prominent decrease of the Ea, with values between 85 and 130 kJ/mol for the mixtures with C-Dolomite and C-Clinoptilolite, which are even lower than the apparent activation energies obtained for pure orange peel at similar conversion degrees. The kinetic constant k also increased with calcination of the catalysts, which shows this thermal treatment proves positive from a kinetic approach.
The calcination treatment showed to shift selectivity of products, with higher content of less-oxygenated compounds found in C-Dolomite and C-Clinoptilolite, compared with the tests where non-calcined minerals were used. On the other hand, hydrogen production was higher for C-Clinoptilolite, while C-Dolomite showed higher selectivity for methane production than its non-calcined counterpart. Therefore, high potential for the use of natural, cheap and abundant materials such as dolomite and zeolite has been found; future studies will focus on the use of different natural minerals, along with simple, chemical modification of the functional groups present in these materials.











 text new page (beta)
text new page (beta)