1. INTRODUCTION
From the last three decades, textile industries are performing a vital role in the socio-economic contribution of Bangladesh. Fatefully, in keeping with the volume and composition, the effluent of textile plants are most polluting amongst all industrial sectors (Sen & Demirer, 2003). Nearly 70 to 150 liters fresh water is required for processing of one kilogram cotton (Allegre, Moulin, Maisseu & Charbit, 2006).
Together with various dyeing chemicals, this huge amount of fresh water is converted into colored and harsh effluent having high temperature, higher chemical oxygen demand (COD) and biochemical oxygen demand (BOD). Bangladeshi textile industries directly discharge their effluents in water channels which severely engendering human health and aquatic environment degradation (Bhuiyan, 2014).
Additionally, various harsh chemicals used in textile processing are liable for waning machinery lifetime by corrosión thus upturns the machine depreciation cost (Karmakar, 1999). Synthetic dyes are allergic, carcinogenic and toxic in nature so the personnel's of industrial dyeing floor are affected in various chronic diseases (Mathur, Bhatnagar & Sharma, 2012). Furthermore, the typical commercial dyeing procedures include various prolong steps (Rahman, Biswas, Mitra & Rakesh, 2014).
The specialists around the world are attempting to build up a cleaner innovation and ecologically supporting methods of cotton coloration. Researchers concluded, sustainable textile processing could be possible either by using highly effluent treatment plant or by using the eco-friendly dyes and chemicals (Blackburn, 2004).
Natural dyes might be promising alternative of synthetics dye for non-allergic, non-carcinogenic, non-toxic, very brilliant, rare color and eco-friendly characteristics. So, the uses of natural dyes are potential viable 'Green chemistry' for avoiding the hazardous synthetic dyes for their various growing environmental and health concerns (Baliarsingh, .Tena, Das & Das, 2013: Islam & Mohammad, 2015; Samanta & Agarwal, 2009: Shaliid-Ul-Islam, Shahid & Mohammad, 2013a, 2013b, 2013c). Moreover, it has no adverse impact on machinery lifetime and equipment depreciation cost.
So, the comparisons of natural and synthetic dyes for cotton coloration are highly required in terms of quality and economic perspective. Researchers had compared the ability of turmeric, acacia bark, eucalyptus and hernia for natural coloration of cotton with reactive dye (Ali, 2007: Umbreen, Ali, Hussain & Nawaz, 2008). Those comparisons revealed that the quality perspective of natural dye was comparable but not economically viable like reactive dye. Regarding cost effectiveness, banana could be a promising dye source.
Among various natural sources banana is most significant for ampie availability. After citrus, Banana is considered as the second biggest created natural product which is contributing around 16% of the aggregate world organic product generation in 129 nations around the globe (Mohapatra, Mishra & Sutar, 2010).
Almost 89 % of banana plant is accounted as waste (Repon, Al Mamun & Islam, 2016). This huge amount of banana waste has no remarkable exploitation at all. In this regards, textile coloration with banana sap will be significant option of effective banana plant waste management. In recent past we have studied, banana floral stem sap has excellent color fastness properties except light fastness (Repon, Al Mamun & Islam, 2016a, 2016b, 2016c). Moreover, regarding comparison with reactive dye it will not be as costly as hernia and turmeric (Ali, 2007; Umbreen, Ali, Hussain & Nawaz, 2008).
So in this study, comparison between reactive and BFS sap for cotton fabric coloration were carried out in terms of the degree of color levelness, color durability, effluent quality and economic feasibility.
2. EXPERIMENTAL
2.1 MATERIALS AND CHEMICALS
2.1.1 Materials
Commercially scoured-bleached 100% cotton knitted single jersey structure fabric having areal density of 175 grams per square meter was collected from "HI-FASHION COMPOSITE TEXTILES LTD", Joydeppur, Gazipur, Bangladesh.
Table 1 depicts the color co-ordinates of the cotton knitted fabric used for this investigation.
2.1.2 Dyes & Chemicals
For natural dye Banana floral stem (Musa sapientum) was collected from Santosh, Tangail-1902, Bangladesh. Reactive dyes such as Remazol Red RR, Remazol Yellow RR, Remazol Blue RR were collected form Dyestar Chemicals ltd, Singapur. All essential and auxiliary chemicals such as Soda ash (Na2C03), Glauber salt (Na2S04. 1OH2O), leveling agent (IRSO), Soaping agent (GASOP-100-RUBY) were procured from Redox Chemicals Ltd, Srilanka. All dyes and chemicals were commercial grade and used without any further purification.
2.2 METHODS
2.2.1 Natural dye extraction
Natural dye in sap form was retrieved form banana (Musa sapientum) floral stem according to our previous work (Repon et al., 2016a, 2016b, 2016c). Briefly, fresh floral stem were cut into one meter pieces using cutlass and then sliced. Sap was extracted from sliced floral stem by roller squeezer machine. The sap solution was filtrated and packed in containers and stored in dark place for avoiding photo degradation.
2.2.2 Dyeing
2.2.2.1 Natural dyeing
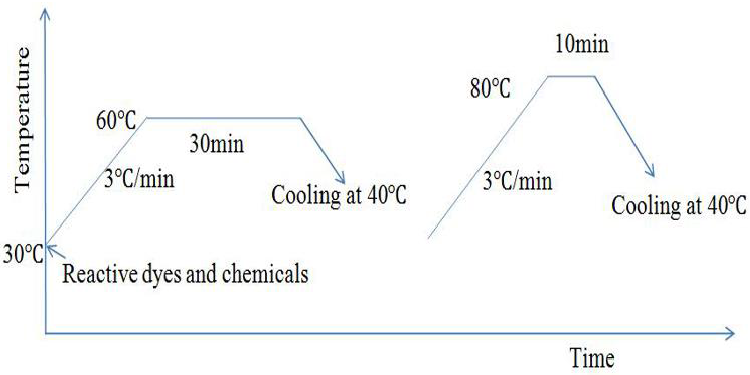
Natural dyeing was performed according to our previous work (figure 1) (Repon et al., 2016a, 2016b. 2016c). Briefly, dyeing had carried out conferring to exhaust method by Infra-red lab sample dyeing machine (XIAMEN RAPID, China) at 100°C for 60 minutes. Then the dye bath was cooled at 40°C and samples were washed at room temperature. Then soaping was performed for removing unfixed dye form the fabric surface by 0.5 g/L ISO standard soap at 80 °C for 10 minutes. In case of dyeing and soaping, material to liquor ratio maintained as 1:20. Samples were squeezed and dried in fíat dryer machine (MESDAN, Italy).
2.2.2.2 Reactive dyeing
Recipe of reactive dyeing is tabulated in table 2 to match the shade with the natural dyed specimens.
Table 2 Recipe of reactive dyeing of 100% cotton knitted scoured blea.ched fabric.
| Chemicals/ | Amount |
|---|---|
| Parameters | |
| Soda ash | 5g/L |
| Glauber salt | 15g/L |
| Leveling agent | 1g/L |
| Remazol Red RR | 0.0119% (on the weight of |
| fabric) | |
| Remazol Blue RR | 0.0096% (on the weight of |
| fabric) | |
| Remazol Yellow RR | 0.0520% (on the weight of |
| fabric) | |
| Material: Liquor | 1:10 |
| Temperature | 60°C |
| Time | 30 minutes |
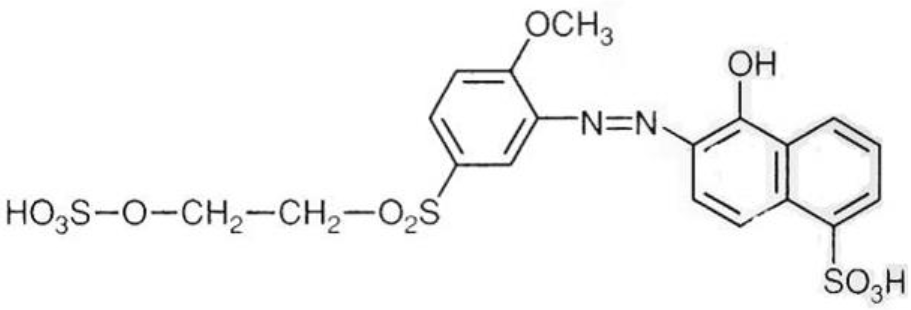
Reactive dyeing was carried out according to the process curve mentioned in figure 2 by maintaining the above recipe. The unfixed dyes were removed from the dyed fabric surface with 1 g/L soaping agent (GASOP-100 RUBY) for 10 min at 80°C.
2.2.3 Determination of dye fixation onto fiber surface
Dye-fiber fixation was observed by FTIR-ATR spectra of scoured bleached cotton, natural and reactive dyed specimens employing FTIR spectrophotometer (PerkinElmer Spectrum Two, UK) machine. Specimens were directly fitted on the respective place of Universal ATR of the machine for dye-fiber bonding characteristics evaluation.
2.2.4 Determination of CMC value
CMC value was determined by the Data-color Spectroflash SF 650X (USA) keeping the setting: Illuminant D65, Médium área view, specular included and CIE 1964 supple-mental standard observer (10° observer). Each sample was folded twice to give an opaque view with four plies and CMC valué was measured automatically (Broadbent, 2001). Natural dyed sample were considered as standard where reactive dyed specimens were batch sample.
2.2.5 Measurement of degree of color levelness
The degree of color levelness was accessed according to our previous work (Repon et al., 2016a, 2016b, 2016c). Briefly, color difference, ∆E value was measured by the following equation 1.
Where, ∆L*= L* sample - L* standard and ∆a* = a* sample - a* standard and ∆b*= b* sample - b* standard. Higher ∆E value denotes the more uneven distribution of the dye onto fabric surface i.e. poor levelness. Based on ∆E value color levelness belongings can be classified as excellent, good, poor and bad (Uddin, 2015).
2.2.6 Determination of Color fastness
Dye-fiber boding stability of the selected dyed fabric was evaluated using various color fastness properties. Color fastness to wash: ISO 105-C06:2010 (AATCC, 2013a), color fastness to rubbing (dry and wet): ISO-105x12:1996 (AATCC, 2013b), color fastness to light: EN ISO 105-B02: 2013 (AATCC, 2006), color fastness to water: EN ISO 105-E01:2013 (AATCC, 1996) and color fastness to perspiration: ISO 105-E04:2013 (AATCC, 2008) were accessed by using grey scale of color change and staining.
2.2.7. Determination of effluent characteristics
The assessment of effluent parameters, viz. biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solid (TDS), total suspended solid (TSS), color and pH valué were done by standard methods (Khan. Islam & Khalil, 2014; ASTM, 2011). Briefly, for measuring COD, both natural and reactive dyeing effluents were kept into COD vial and set it into COD reactor at 150°C. After 2 hours, the vial was put off and cooled at room temperature. Then the COD value of specimen was accessed using UV spectrophotometer by comparing with reference COD vial. For accessing BOD, distilled water and distilled water mixed effluent were taken in two sepárate BOD oxitop and kept those into incubator at 20°C for five days. Then the BOD valué was accessed by comparing the dissolved oxygen of puré and impure samples. To measure the TSS and TDS, a piece of filter paper and clean glass beaker were dried and weighed. One liter water was engaged to filter. Then the filter paper was dried carefully and reweighed. Here, the change in weight of filter paper is the total suspended solids. Similarly, the fiftered water was taken into dried clean glass beaker and placed it into an oven to evapórate the water. After cooling, the weight of beaker was taken to determine TDS.
Henee, the change in weight of clean beaker is TDS. Color was measured by ASTM D1209 method using Platinum Cobalt scale. The pH valúes of natural and synthetic dyeing wastewater water were accessed by pH meter.
3. RESULT AND DISCUSSION
Price of raw materials, dyes & auxiliary, processing cost, power & energy and personnel related cost had considered for cost estimation.
3.1 FIXATION OF DYE ONTO FIBER SURFACE
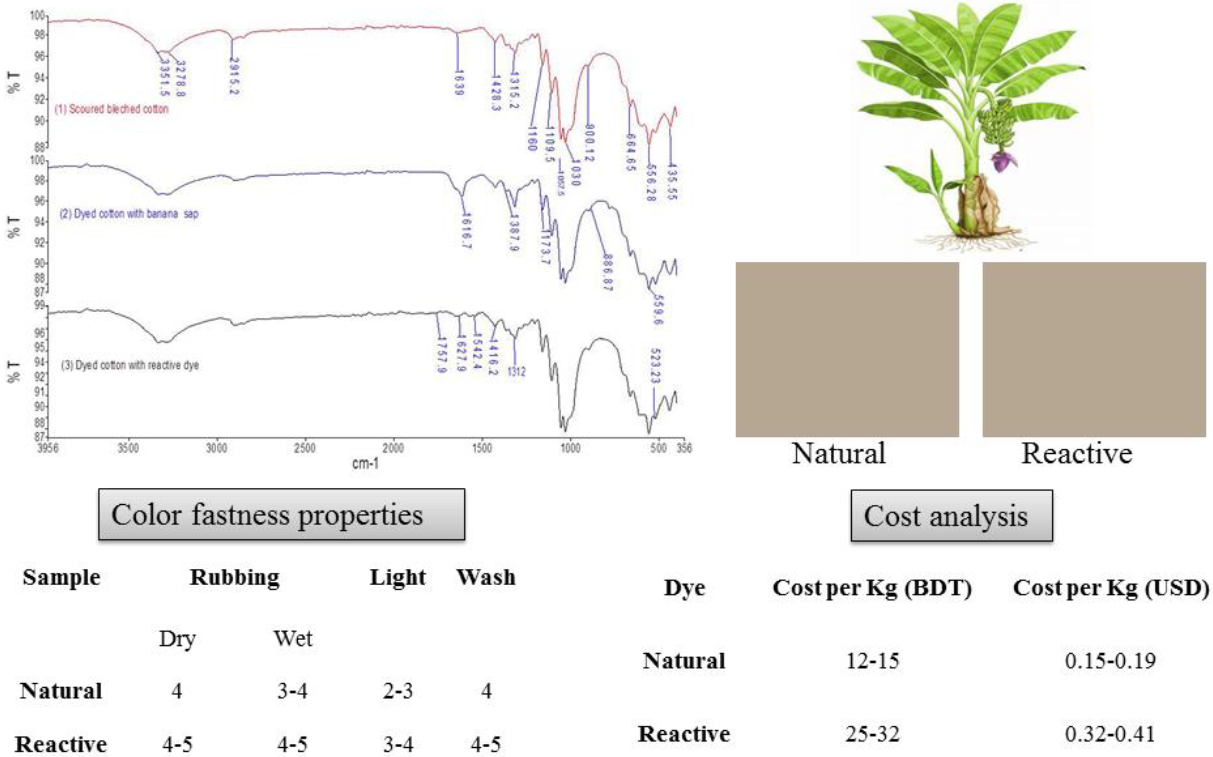
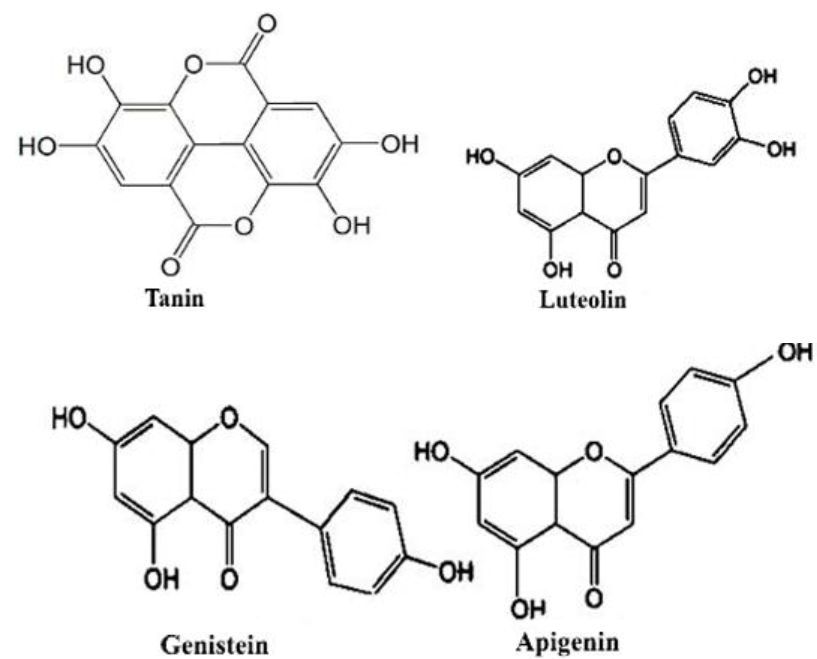
Figure 3 indicates the FTIR-ATR spectra of scoured bleached (1), natural (2) and reactive dyed (3) cotton fabric. Table 3 denotes the adsorption bands for imparting color into fabric. Various peaks appears approximately at 435-560cm-l, 620-680 cm-1, 1000-1210 cm-1, 1315-1387, 1420, 1640-1660 cm-1, 2838-2915 cm-1, and 3270-3330 cm-1 for OH out of plañe bending, glycoside linkage and asymmetric out of phase ring stretch of C1-O-C4, C-H bending, di-alkyl ether linkage (C-O-C) and -C-0 symmetric stretching of cellulose, hemicellulose and lignin content , absorbed water vibrations, -CH stretching vibrations and corresponding to hydrogen bonded -OH stretching vibrations respectively (Miller &Wilkins, 1952). For natural dyed specimens (2) major changes were appeared at 703.27, 780.32, 1173.71 and 1616.19 cm1 due the presence of inorganic salts in banana floral setm sap into viz. potassium chloride, sodium phosphate and magnesium chloride respectively (He, et al., 2007: Monteiro, Margem, Loiola, Assis & Oliveira, 2014). Peaks at 1056.62 correspond C-H and C-O deformation band (Ibrahim, Dufresne, El-Zawawy & Agblevor, 2010) and 1396 cmJ was responsible for CH deformation of -CH2-(Bashar, Siddiquee & Khan, 2015; Khandare, Kabra. Tamboli & Govindwar, 2011). For natural dyed fabric (2), stronger bands were seen at peaks 431.4, 521.4, 607.9, 665.4, 1160.2, and 1427cnr1 correspondingly than control (1) by decreasing transmittance % due to presence of inorganic salts, tannin, flavonoid, lignin, etc (figure 5) (Ibrahim et al., 2010).
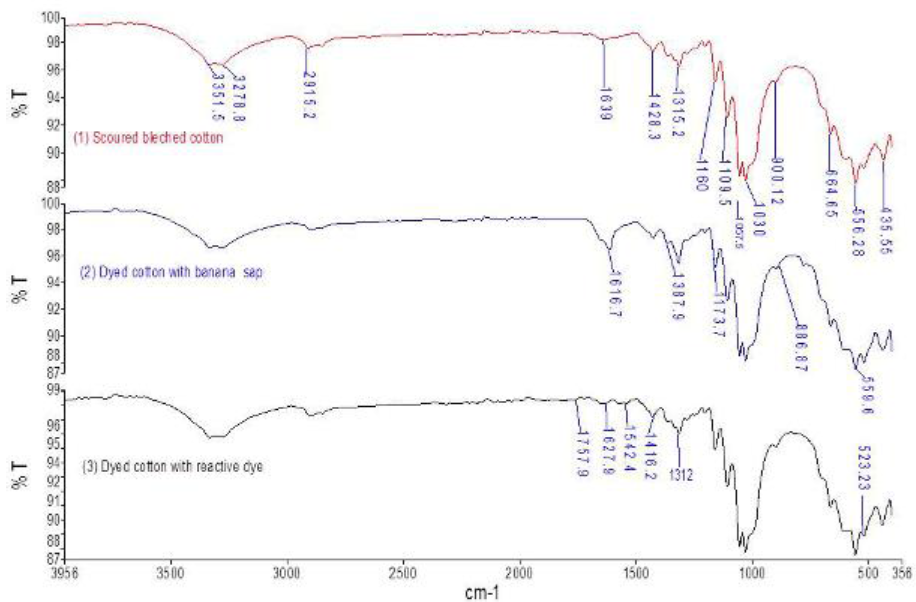
Fig. 3 FTIR-ATR, spectra of cotton (1) scoured bleached, (2) dyed with banana sap, (3) dyed with reactive dye.
Table 3 FTIR adsorption bands for imparting color into fabric.
| Position (cm -1) | Suggested band origin |
| 1727.9 | C=0 of ketone |
| 1542.4 and 1627.9 | N-H deformation |
| 1100-1400 | Ether linkage, -C-O symmetric stretching |
| 1056.62 | C-H and C-O deformation |
| 1396 | CH deformation of -CH2- |
For reactive dyed samples (3), weak peaks were observed at HOO-MOOcm-1 for ethar linkage between reactive dye and cellulose (Bashar et al., 2015). It is evidient that peaks 1542.4 and 1627.9 are responsible for N-H deformation of vinyl sulphone (β sulfato ethyl sulfone group) reactive dye (figure 4) . Band at 1727.9 was looked for C=0 of ketone (Khandare et al., 2011).
3.2. CMC VALUE
Table 4 embodies the CMC report considering natural as standard and reactive as batch sample. Figure-6 denotes the photographs of BFS sap dyed and Reactive dyed fabric. CMC decisions were accepted as "Pass" for illumination D65 10 degree where difference (DE CMC) was 0.45. Difference in lightness, redness, yellowness, chroma and hue angle were detected as 1.19, -0.05, -0.03, -0.05 and 0.04 respectively. The sample dyed with reactive dye was resembled as slightly lighter than the natural dyed specimens. More green and more blueness were also observed in reactive dyed sample but less dye saturation. Metamerism was not observed in prescribed settings.
Table 4 CMC value
| III/Obs | CMC Decisión | CMC DE | DL*1 | Da*2 | Db*3 | DC*4 | DH*5 | Metamarism Index | |
|---|---|---|---|---|---|---|---|---|---|
| D65 lODeg | Pass | 0.45 | 1.19 | -0.05 | -0.03 | -0.05 | 0.04 | ||
| msTL 84-10 | Fail | 1.24 | 1.17 | -0.88 | -0.41 | -0.70 | 0.67 | 0.91 | |
| A 10 Dcg | Fail | 1.18 1.20 | -0.49 | 0.39 | 0.09 | 0.62 | 0.61 |
1 Difference in Lightnees, 2 Difference in Redness, 3 Difference in Yellowness, 4 Difference in Chroma, 5 Difference in Hue
3.3 DEGREE OF COLOR LEVELNESS
Homogeneity of dye molecule distribution onto the fiber surface essentially indicates the levelness of color. The natural and reactive dyed samples were showed excellent color levelness (table 5) . Natural dyed specimens were 88% level as compared to reactive dyed samples.
Table 5 Degree of color levelness.
| Samples Types | ∆E values of dyed samples | Average ∆E | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R-1 | R-2 | R-3 | R-4 | R-5 | R-6 | R-7 | R-8 | R-9 | R-10 | ||
| Batch readings | |||||||||||
| Natural | 0.292 | 0.038 | 0.067 | 0.029 | 0.072 | 0.047 | 0.048 | 0.017 | 0.014 | 0.069 | |
| Reactive | Standard | 0.123 | 0.129 | 0.056 | 0.032 | 0.098 | 0.068 | 0.031 | 0.050 | 0.019 | 0.061 |
In reactive dye bath, electrolyte and leveling agent are usually used to overeóme the inherent inharmonious spreading of dye molecules onto the fiber surface. Conversely, no leveling agents were required for natural dyeing to produce similar color levelness (Repon et al., 2016a, 2016b, 2016c). That means over all more level color were yielded for BFS sap.
3.4 COLOR FASTNESS
Table 6 represents the results of color fastness to wash, water and perspiration andshows the color fastness to rubbing and light of natural and reactive dyed samples respectively. Excellent upshots were recorded for reactive dyed cotton samples regarding wash, water and perspiration (both in acid and alkali) fastness.
Table 6 Color fastness to wash, water and perspiration.
| Color | Typcs of | Change in Color | Stainiug | in Color | ||||
| Fastness | dye | Acétate | Cotton | Polyainidc | Polycstcr | Acrylic | Wool | |
| Wash | Natural | 4 | 4-5 | 4 | 4 | 4-5 | 4-5 | 4 |
| fastness | Reactive | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 |
| Water | Natural | 4 | 4-5 | 4-5 | 4 | 4-5 | 4-5 | 4 |
| fastness | Reactive | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 |
| Acid | Natural | 4 | 4-5 | 4 | 4-5 | 4-5 | 4-5 | 4-5 |
| ratio | Reactive | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 |
| £< Alkali 0) | Natural | 4 | 4-5 | 4 | 4 | 4-5 | 4-5 | 4-5 |
| Reactive | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 |
The natural dyed samples showed very good rating of color fastness to washing, water and perspiration. Regarding wash fastness, slightly color changes were observed onto cotton, polyamide and wool portion. Concerning water fastness, little staining was witnessed onto polyamide and wool part of multi fiber fabric. Color staining was appeared onto cotton for acid perspiration. For alkaline perspiration color staining was looked onto cotton and polyamide.
Inherently, both reactive dye and cotton deliver anión into water thus repulse one another. The positive rings onto cellulose surface are generated due to the addition of electrolyte in the dye bath and stronger covalent bond formed between cellulose and reactive vinyl sulphone) P sulfato ethyl sulfone) group. Consequently, reactive dyed samples exhibit excellent fastness grading (Repon et al.. 2016c). In contrast, BFS comprises of many organic and inorganic compounds (Monteiro et al., 2014). From the máximum absorption wavelength (Amax= 350nm), it can be presumed that the color appeared in the dyed samples is liable for flavonoids (Pinheiro & Justino, 2012). Tannin of BFS dye has excellent dye molecule cross linking performance via co-ordination bond onto the cellulose surface that was conformed from the FTIR-ATR spectra. So, almost equivalent color durability (washing, water and perspiration) were observed for natural dyed cotton alike reactive dye. Excellent behaviors were noticed for dry and wet rubbing fastness (Table 7) of reactive dyed cotton. For natural dyeing, dry and wet rubbing fastness were graded as very good i.e. 4 and modérate i.e. 3-4 respectively. These observations also express that for stronger binding ability between reactive dye and fiber is favorable for excellent color durability to rub. Conversely, the weak co-ordination bond between natural dye and fiber exhibited modérate upshot (Ali, 2007).
Regarding light fastness, the ratings were recorded as 2-3 i.e. poor to fair for natural dyed specimens. Oppositely, 3-4 i.e. fair to modérate ratings were appeared for reactive dye samples. Higher light fastness (Table 7) properties of reactive dyes can be attributed for more intra molecular H bond. This enhances the stability of the dye compound by reducing the electrón density at chromospheres. Therefore, the sensitivity of dye toward photochemical oxidation was reduced (Ali, 2007). Alternatively, such strong interaction is not present in the bonding between natural dye and cotton fabric. So the natural dyed specimens were exhibited inferior light fastness property than reactive dyed specimens. Thus, both natural dyed and reactive dyed samples were comparable for color durability without light fastness properties (Ali, 2007).
3.5 EFFLUENT PARAMETERS
Table 8 shows the various parameters of effluent for natural and reactive dyeing separately.
Table 8 Paramcter of Effluent for natural and reactive dyeing.
| Parameters | Reactive dye | Natural dye | DoE standard |
| BOD | 153mg/L | > 1,000 mg/L | 50mg/L |
| COD | 427mg/L | 7,303 mg/L | 200mg/L |
| TDS | 1210 mg/L | 9,800 mg/L | 2100 mg/L |
| TSS | 112 mg/L | 1,490 mg/L | 150 mg/L |
| Color | 2,10()Pt Co | 20,920Pt Co | 150 Pt Co |
| PH | 8.5 | 6.8 | 6-9 |
BOD, COD, TDS, TSS, Color and pH of reactive and natural dyeing effluent were found as 153, 427, 1210, 112 mg/L, 2100 Pt Co and 8.5 and > 1000, 7303, 9800, 1490 mg/L, 20920 Pt Co and 6.8 respectively. Almost all effluent parameters of reactive dyeing were lower than the DoE standard except color and pH. This is oceurred for using of less amount and few auxiliary chemicals compare to commercial dyeing process (Khan, Islam & Khalil, 2014). Though valúes of effluent parameters of natural dyeing were higher than DoE standard but it is not a matter of headache for environmental pollution for their biodegradability aspect (Khan et al., 2014). The higher valué of TDS and TSS of natural dye effluent were ensued because of the co-existence of metal and organic compound in banana sap. Lower color contení appeared in reactive dye bath for greater absorption% of dye for improved affinity of cotton towards reactive dye molecules and addition of electrolyte. Conversely, higher color content observed in the bath of natural dyeing for lower exhaustion of molecules and zero use of auxiliary chemicals for improving dye-fiber interaction.
3.6 COSTINO
Table 9 shows the comparative cost of natural and reactive dyeing process for one kg fabric. In case of producing similar shades, cost of natural dyeing was noticed much lower than reactive dyeing.
Table 9. Cost of natural and reactive dyeing per Kg of knitted cotton fabric.
| Types of dye | Cost per Kg (BDT) | Cost per Kg (USD) |
|---|---|---|
| Natural | 12-15 | 0.15-0.19 |
| Reactive | 25-32 | 0.32-0.41 |
Because, after harvesting of banana fruit, banana plants leads huge amount (89%) of waste (Repon et al.. 2016a). Moreover, no auxiliary chemicals were used in natural dyeing bath. Therefore, only processing cost and negligible amounts of extraction charges are applicable. Conversely, reactive dyeing was associated with more dyes, auxiliary chemicals and processing costs. Dyeing cost of cotton fabric with banana floral stem sap were also less than that of henna leaves, acacia bark and turmeric bark (Ali, 2007; Umbreen, Ali, Hussain & Nawaz, 2008). Furthermore, as BFS dye is fully safe so it has zero effluent treatment cost. Interestingly, we didn't use and additional water in main dyeing process. Only few amount of water was engaged for removing unfixed dye from the fabric surface. So negligible water treatment plants cost is applicable for the BFS dye. Additionally, the process of cotton dyeing with BFS sap didn't include various prolong steps as typical commercial process (Rahman, Biswas, Mitra & Rakesh, 2014) which reduces the dyeing time wittily. Furthermore, various harsh chemicals in reactive dyeing are responsible for machine corrosión that leads machine depreciation cost (Karmakar, 1999). In this regards, BFS sap is fully safe for machinery and it will reduce the equipment's depreciation cost ingeniously. Thus, BFS dye reduced dyeing cost almost half of the synthetic dye.
4. CONCLUSION
Comparative analysis between natural and reactive dyeing discloses that natural dyed specimens have excellent color levelness and durability properties. No additional water was used in main dyeing process of cotton with banana floral stem sap. Only, few amount of water was engaged for removing unfixed dye from the fabric surface. Thus, economic viability was assured in both water and effluent treatment plants. Additionally, it is ecofriendly and machine friendly as well. In consequence, cotton dyeing with banana floral stem sap requires almost cost than reactive dyeing. From the view of quality, BFS sap was exhibited excellent color fastness properties except light fastness. So it can be easily deployed where light fastness is not prime headache. Finally, it can be concluded that from the sustainability, quality and economic perspective, BFS sap is easily acceptable as alternative of reactive dye.
List of Abbreviations and Symbols
| BFS= Banana floral stem |
| COD= Chemical oxygen demand WI= Whiteness Index BI= |
| BOD= Biochemical oxygen demand |
| WI= Whiteness Index |
| BI= Brightness Index |
| L*= Lightness |
| a*=Redness |
| b*=Yellowness |
| c*=Chroma |
| H=Hue |
| DoE= Depart of Enviroment |
| TDS=Total disolved solid |
| TSS= Total suspended solid |
| CIE: Commission Internationale de l'éclairage |
| CMC= Color Matching Committee |
| Na2CO3=Soda ash |
| Na2SO4. 10H2Q= Glauber salt |
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.











 text new page (beta)
text new page (beta)