1. INTRODUCTION
Limited supplies of petroleum-based fuel and a massive impact on atmospheric CO2 levéis as a result of its burning processes have encouraged intense interest to a renewable and environmentally-friendly fuel.
Discovering adequate supplies of renewable and clean fuel for the future survival is the most daunting challenges since they significantly determine economic prosperity, global stability, and quality of life. This phenomenon induces appealing debate and questions over new fuel choices, resulted from new raw materials, to partly complement or entirely substitute present petroleum-based fuels. Biomass has recently turned out to be a topic of passionate interest.
Yet, determining to which biomass with their yield promises for biofuel candidates appears as most gorgeous challenges. The use of first-generation biofuels, which have focused on higher plants such as sugarcane, corn, oil palm, soybean, algae, and others, raises new issues associated with the global food security and food markets or the loss of ecosystems. Second-generation biofuels resulted from lignocellulosic biomass such as forest and agriculture residues, as well as nonfood vegetable feedstock, can address some of the above problems; nevertheless, there is an alarm over competing land use or required land use changes (Brennan & Owende, 2010). While most bioenergy alternatives miss opportunities as replacement fossil fuels due to various constraints, aquatic microorganism-based biofuels have a good prospect to genérate a significant amount of renewable energy without disruptions. Based on current knowledge and technology projections, microalgae are taken into account to be third-generation biofuels which are technically feasible as the alternative energy reserves, and they are without major drawbacks compared to the first and second generation biofuels (Brennan & Owende, 2010)
Microalgae have become one of the most important renewable energy sources owing to their flexible cultivation and neutrality towards the natural environment. Some species of microalgae can be cultured in brackish water on non-arable land (Brennan & Owende, 2010; Schwenk et al., 2013), and consequently, they may not bring in land-use change. Cultivation of microalgae could be conducted under non-controlled conditions, open bioreactors (Da Rosa, Carvalho, Goldbeck, & Costa, 2011), as well as open ponds (Crowe et al., 2012; Sánchez, González, Maceiras. Cancela, & Urrejola, 2011), and microalgae use sunlight as the primary source of energy. Therefore, a non-controlled cultivation is easy to build and opérate with low initial investment (Da Rosa et al., 2011). Producing and harvesting microalgae have uses in wastewater treatment and CO? fixation (Christenson & Sims, 2011; Wang, Li. Wu, & Lan, 2008), and by naturally fixing atmospheric CO? via photosynthesis they are ten times more efficient than terrestrial plants (Cantrell, Ducey, Ro, & Hunt. 2008). Henee, it would reduce environmental impaets. Microalgal biomass is arranged by 50% carbón by dry weight, and its element is originated from carbón dioxide (Sánchez Mirón et al., 2003). Producing 1 kg of algal biomass requires 1,83 kg of CO? (Yusuf, 2007); thereby, microalgae cultivation is critical for global warming mitigation (Sukarni, Sudjito, Hamidi, Yanuhar, & Wardana, 2015). Moreover, algae cultivation does not undermine the production of food, fodder and other producís derived from crops . Microalgae can be a year-round harvest (Phukan, Chutia, Konwar, & Kataki, 2011) providing a reliable and continuous supply of biomass. They have higher annual biomass productivity on an área basis due to their fast growth rate and high biomass yield . Thus, they will be prospective as essential future energy reserves to address the fossil fuels problems.
Exploring the feasibility of microalgae for future biomass fuel feedstock obligates to investígate their biomass abundance as well as their physicochemical properties. The algal biomass productivity will ensure their continuous supply of biomass fuel feedstock. The algal physicochemical properties are also critical parameters because they will provide a strong effect on the combustión characteristics. The latest references associated with the microalgal biomass fuel specify that very few studies have been focused on the microalgal biomass characterization. Phukan, Chutia, Konwar, and Kataki (2011) have investigated the potential bio-energy feedstock of Chlorella sp.MP-1 regarding its physical and chemical characteristics by using bomb calorimeter, thermogravimetric analysis (TGA), CHN analyzer and Fourier transform infrared (FTIR) spectroscopy. Sukarni, et al. (2014) have studied the potential of marine microalgae Nannochloropsis oculata concerning its abundance and physicochemical properties. However, the potential of Isochrysis galbana as a biomass fuel feedstock has not been explored yet. In connection with the broadening opportunities for seeking alternative energy sources, exploring the potential of Isochrysis galbana as solid fuel feedstock is very critical. Henee, for optimizing the usefulness of this biomass associated with the propriety of combustión technology and the potential future applications, it requires further in-depth research.
This work endeavored to fill partially in the gap, by investigating the properties of Isochrysis galbana in accordance with its prospect as biomass fuel feedstock. The samples were characterized in relation to their biomass growth rate, heating valué, moisture and ash content, as well as their chemical composition. The cellular macromolecular content of biomass and residue have also been evaluated by using FTIR spectroscopy. The study also analyzed the biomass thermal characteristic under a nitrogen and air atmosphere.
2. MATERIALS AND METHODS
2.1 SPECIES CULTIVATION AND GROWTH EVALUATION
The marine microalga Isochrysis galbana species was obtained from the Center for Development of Brackish Water Aquaculture (Balai Besar Pengembangan Budidaya Air Payau-BBPBAP), Jepara, Indonesia. Eight Erlenmeyer flasks with seawater médium were used to cultívate the species. During the cultivation process, three-time measurement was performed for salinity, pH, temperature, and dissolved oxygen of the médium, i.e. at the beginning, middle and end of the period. The fertilizer, whose composition was shown in Table 1, enriched the cultivation médium. The species' culturing duration was eight days.
Tabla 1 The Walne fertilizer composition.
| Substances | Composition (ppm) |
|---|---|
| NaNOa | 100 |
| NaH2PO4 | 20 |
| H3BO3 | 33.6 |
| NaEDTA | 45 |
| FeCla | 1.3 |
| MnCl2 | 0.36 |
| Na2SiOa | 10-80 |
| Vitamin B12 | 0.001 |
The daily growth evaluation was conducted during the cultivation period by calculating the number of cells in the cultivation médium using hemocytometer coupled with an optical microscope (CH2 Olympus Optical Co. Ltd, Tokyo, Japan). The sample calculation was done every day at 10:00 AM for three-time measurement and then averaged. The specific growth rate (¡i in unit d-1) of the biomass was specified according to Sukarni, et al. (2014) and calculated as following:
Where C n is the cell number density (cells mí4), t is time (d), C t is cell density at time (t), and C 0 is cell density at the start of the exponential phase (t0). The doubling time (d), i.e. the time required to double the cell, is determined as the following:
2.2 HARVESTING, DRYING AND PULVERIZING
The biomass' harvesting process has been carried out by precipitating the biomass suspensión with caustic soda and then filtering and washing it twice with distilled water. The precipitated materials were subsequently sun-dried for three days. The fine particles of biomass were attained by crushing the biomass chunks with a mortar. A vacuum desiccator was employed to store the pulverized samples.
2.3 PROXIMATE ANALYSIS AND HIGHER HEATING VALUE (HHV) CALCULATION
The proximate analysis, which determines contents of moisture, volatile matter, fixed carbón and ash in the biomass samples, was performed by an experiment in according to the test method of ASTM D 3173-11, ASTM D 3175-11, ASTM D 3172-13 and ASTM D 3174-12, respectively. The adiabatic bomb calorimeter was employed to determine the heating valué of biomass. The sample about 0.5 g was used for 10 min with 99.5% puré oxygen.
2.4 CHEMICAL COMPOSITION ANALYSIS
The chemical composition of the algal biomass was determined by an Energy-dispersive X-ray (EDX) spectrometry (FEI, Inspect-S50) coupled with X-ray microanalysis capabilities (AMETEK EDAX TSL). Minimizing the surface electrón charging effect of samples was achieved by gold-coating so that the distort images did not take place during the samples' evaluation.
The macromolecular content of biomass, which represented the presence of carbohydrate, protein, and lipid in the algal biomass, was evaluated by using Shimadzu FTIR spectroscopy. Before measuring process, the potassium bromide (KBr) powders were mixed with the samples and then mixed samples were pressed into tablets. The scanning of samples was conducted from 400 to 4000 cnr1. Likewise, investigation of macromolecular content change after the thermal processes of biomass had performed by the similar method.
2.5 THERMAL ANALYSIS
A METTLER TOLEDO TGA/DSC1 simultaneous analyzer was employed in the current study to continuously monitor mass changes of Isochrysis galbana sample as a result of thermal degradation in the nitrogen and air environment, respectively. The sample followed a fixed linear heating program of 10 °C min1. The small samples (approximately 10 mg) were loaded into an AI2O3 ceramic crucible and then inserting into the reactor, which subsequently was heated up from ambient temperature to 1000 °C. During the process of pyrolysis and combustión, the TGA furnace was flowed by nitrogen as an inert and air atmosphere as an oxidizer, where each test was supplied with a constant flow rate of 100 mi min"1. The continuous on-line records of mass loss as a function of temperature and time were obtained to plot the thermogravimetric analysis (TGA) curve as well as the derivative thermogravimetric (DTG) one.
3. RESULTS AND DISCUSSION
3.1 THE CULTURE CONDITIONS AND BIOMASS PRODUCTIVITY
The appropriateness of microalgae for biomass fuel candidates, in terms of the sustainability, is largely determined by the biomass abundance. Henee, microalgae productivity is one of the most important parameters which must be examined. In this work, the Isochrysis galbana culture condition was shown in Figure 1. Its environmental parameters, represented by the salinity, pH, temperature and dissolved oxygen (DO) of the médium, were depicted in Table 2. The microalgae are very sensitive to their growth environment. Henee, these environmental parameters are interesting in relation to both biomass productivity and properties.
Table. 2 The environmental parameters of culture médium. The error represented the standard deviations from eight measurements of eight samples.
| Cultivation periods (d) | Salinity (%o) | pH | Temperature (oC) | DO (mg l1) |
|---|---|---|---|---|
| 1st | 25.25 ± 1.28 | 8.56 ± 0.50 | 24.13 ± 0.48 | 7.24 ± 0.26 |
| 4th | 38.75 ± 1.98 | 8.69 ± 0.46 | 23.88 ± 0.42 | 7.33 ± 0.15 |
| 8th | 40.38 ± 1.60 | 9.00 ± 0.00 | 24.31 ± 0.27 | 7.53 ± 0.11 |
The Isochrysis galbana biomass abundance during the eight days cultivation period was shown in Figure 2.
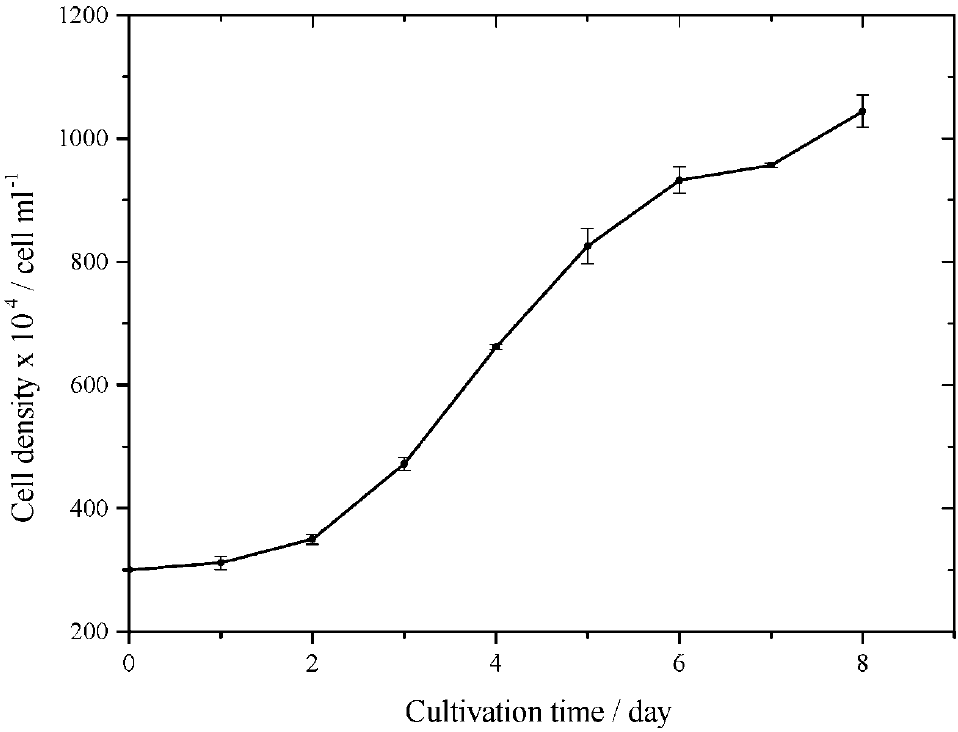
Fig. 2 The biomass abundance of Isochrysis galbana during eight days cultivation. The error denoted the relative standard error of mean from three replications of each sample.
It was started at around 3 x 106 cells mb1 under the initial cultivation, and then the density reached 1044 x 106 cells mb1 under the end one. In terms of growth kinetic, Isochrysis galbana had 0.18 d-1 for the specific growth rate and 3.85 d for the doubling time. It meant that 0.26 cell was double, or división per day and average increases in biomass were 9.3 x 10° cells d-1. It was clearly that Isochrysis galbana had good biomass productivity. The productivity of Isochrysis galbana compared with other species cultured under different conditions and media was presented in Table 3. In the growth kinetics point of view, it could be observed that Isochrysis galbana had higher productivity than Chlorella vulgaris and of the Nannochloropsis oculata did, in which both were grown in the Bold's Basal médium and the Guillard f2 one (Converti, Casazza, Ortiz, Perego, & Del Borghi, 2009), respectively. Additionally, the Isochrysis galbana productivity equals Dunaliella salina cultured in outdoors-closed tubular photobioreactor (García-González, Moreno. Manzano, Florencio, & Guerrero, 2005) and almost equals Nannochloropsis gaditana cultivated in the indoor system (Selvakumar & Umadevi, 2014). Table 3 also indicated that Isochrysis galbana productivity is less than Chlorella protothecoides (Illman, Scragg, & Shales, 2000), Nannochloropsis oculata (Sukarni et al., 2014) and Scenedesmus obliquus (De Moráis & Costa, 2007), in which all three species were cultured in a low nitrogen Guillards marine médium, a traditionally natural open pond, and tubular photobioreactor, respectively. Due to the sensitivities of microalgae to the environmental culturing (Alkhamis & Qin, 2013; Kaplan, Cohén, & Abeliovich, 1986), its comparison could not exactly show an algal biomass productivities benchmark, but at least could provide an overview for future algae cultivation. Therefore, Isochrysis galbana productivity may still be improved by the environmental conditioning, with regard to the culture médium and nutrients engineering.
Table. 3 The kinetic growth rate comparison of various microalgae species.
| Species | Cultivation | µ(d-1) | t d (d) | Ref |
|---|---|---|---|---|
| Dunaliella salina | Outdoors- closed tubular photobiorector | 0.18 | 3.85 | (1) |
| Scenedesmus obliquus | Tubular photobireactor | 0.22 | 3.15 | (2) |
| Chlorella vulgaris | Bold's Basal medium | 0.14 | 4.95 | (3) |
| Nannochloropsis oculata | The Guillard f2 medium | 0.07 | 9.90 | (3) |
| Nannochloropsis gaditana | Indoor Culture | 0.199 | 3.48 | (4) |
| Chlorella protothecoides | Low nitrogen Guillards Marine médium | 0.27 | 2.6 | (5) |
| Nannochloropsis oculata | Open pond | 0.27 | 2.6 | (6) |
| Isochrysis galbana | Erlenmeyer flask Indoor Culture | 0.18 | 3.85 | This study |
(1) García-González et al. (2005); (2) De Moráis & Costa (2007); (3) Converti et al. (2009); (4) Selvakumar & Umadevi (2014); (5) Illman et al. (2000); (6) Sukarni et al. (2014)
3.2 THE PHYSICOCHEMICAL PROPERTIES
The main physical properties of solid fuels were the moisture content, volatile matter, fixed carbón, and ash, where overall parameters are ordinary known as proximate valúes. Moreover, the most critical parameter in relation with the biomass fuel was the heating valué. The Isochrysis galbana proximate results, as well as the heating valué and their comparison with another biomass, were summarized in Table 4.
Table. 4 The physical properties of some biomass.
| Biomass | Proximate Analysis | Ref | |||||
|---|---|---|---|---|---|---|---|
| Moisture | VM | FC | Ash | HHV | |||
| (wt%) | (wt%) | (wt%) | (wt%) | (MJ kg-1) | |||
| Terrestrial plant | Bagasse | 70.90* | 7.00* | 22.10* | 14.258 | (1) | |
| Sugar cane Straw | 76.20* | 14.60* | 9.20* | 17.19 | (2) | ||
| Cotton residue | 72.80* | 20.59* | 6.61* | 16.90 | (2) | ||
| Aquatic biomass | Chlorella sp. MP-1 | 6.8 | 72.19 | 15.08 | 5.93 | 18.59 | (3) |
| Chlorella vulgaris | 55.3* | 34.35* | 10.28* | 21.88 | (4) | ||
| Chlorella vulgaris | 18 | (5) | |||||
| Chlorella minutissima | 21 | (5) | |||||
| Chlorella vulgaris | 9.1 | 37.3 | 5.0 | 48.6 | (6) | ||
| Enteromorpha clathrata | 13.30 | 41.82 | 7.79 | 37.09 | 7.89** | (7) | |
| Sargassum natans | 10.46 | 48.85 | 11.60 | 29.09 | 8.68** | (7) | |
| Nannochloropsis oculata | 3.99 | 64.76 | 7.76 | 23.50 | (8) | ||
| Nannochloropsis oculata | 67.45* | 8.08* | 24.47* | 16.80 | (8) | ||
| Isochrysis galbana | 12.98 | 40.10 | 7.47 | 39.45 | This study | ||
| Isochrysis galbana | 46.08* | 8.58* | 45.33* | 16.22 | This study | ||
* indicated VM, FC and Ash in dry basis (db), other demonstrated on an air-dried basis (adb)
**indicated lower heating value (LHV) in unit of MJ kg-1
(1) Parikh, Channiwala, & Ghosal (2005); (2) Yin (2011); (3) Phukan et al. (2011); (4) Chen, Ma, & Liu (2011); (5) Illman et al.(2000); (6) Agrawal & Chakraborty (2013); (7) Wang, Jiang, Han, & Liu (2009); (8)Sukarni et al. (2014)
Table 4 indicated that Isochrysis galbana had nearly 12.98% of moisture content. This moisture would be released in the first stage of the thermal conversión process. As shown in Table 4, the moisture content of biomass varied widely, depending largely on the type of biomass. Moreover, the biomass moisture content was also strongly influenced by the drying process and storage mode. Comparing sun-dried Isochrysis galbana to another biomass, it clearly that it had a relatively higher moisture content. This result pointed out that Isochrysis galbana which sun-dried for three days appears less dry as the fuel feedstock. Therefore, the longer sun-dried process or other drying methods may be needed for reducing this moisture content.
The moisture content was the critical parameter of solid fuel owing to its impact on the overall performance of combustión processes. Because releasing moisture at the initial step of combustión required an amount of energy, biomass with higher moisture content would take more energy for drying out, and subsequently for heating their vapor. It would reduce the máximum potential energy resulted during the biomass combustión processes and their overall efficiency, as well. As the moisture reléase needs a quantity of energy in the initial step of combustión, it also determines the evaporation time, so that higher moisture content implies more residence time in the chamber. The increasing residence time of biomass in the chamber before the occurrence of gasification and combustión, this leads to required larger equipment during combustión processes and consequently affected the size of the entire combustión system. Moreover, moisture in biomass also affected both burning reaction rate and reaction zone thickness which directly determined the forming of gas emission and influenced the devolatilization rate, respectively.
After releasing moisture during the thermal conversión process, as a result of the increasing temperature, a most organic element of the biomass underwent softening, swelling, and subsequently thermal cracking. It led to the vaporized material known as volatile matter. This volatile matter would determine both initial ignition and burnout time in the first step of combustión processes. Finding out volatile matter amount was needed to determine the quantity accuracy of supplied air to the combustión zone where volátiles was released. The sufficiency of air supplied to this zone affected the combustión efficiency. As shown in Table 4, the volatile content of Isochrysis galbana was 46.08% on the dry weight basis. This volatile content was lower than that of both the terrestrial plant and the other aquatic biomass. This lower volatile content implied on its combustión reactivity. Owing to volatile matter was the most flammable components of biomass, the lower volatile meant harder biomass to be burned or less in reactivity. Conversely, the higher volatile in the fuel, the easier the ignition would be. Therefore, Isochrysis galbana with less volatile matter content was more difficult to be burned than other tabulated biomass.
Non-combined carbón, generally known as fixed carbón, is the portion left over once the volatile matter is entirely released, apart from ash and moisture. That carbón was burned to form char. The portion of volatile matter and fixed carbón in the biomass, which expressed as VM/FC ratio, is the critical parameter determining the reactivity of biomass. The higher this ratio was, the easier the biomass was ignited. Conversely, lower ratio made the biomass ignition more difficult. Table 4 showed that the fixed carbón content of Isochrysis galbana was 8.58% on dry weight basis (7.47 %, adb). Its content was higher than that of bagasse and Chlorella vulgaris (Agrawal & Chakraborty, 2013), and comparable to Enteromorpha clathrata and Nannochloropsis oculata biomass. It meant that Isochrysis galbana was less in reactivity and had higher residence stage until the combustión was completed compared to both of bagasse and Chlorella vulgaris. Nevertheless, its fixed carbón content significantly determined the heating valué, and it impacted on the heat generation during the combustión processes.
The remaining mass left after the complete combustión of char was ash. It contained inorganic materials, which were incombustible parts of the fuel. These materials were derived from a mineral fraction of the biomass origin. The ash content of biomass fuel was critically associated with the appropriateness of combustión and gas-cleaning technology choices. Furthermore, the residual handling mode and overall operating costs of the combustión system were greatly determined by the ash content of the fuel. Henee, biomass fuels with low ash content were desirable. Table 4 indicated that Isochrysis galbana, whose ash content was 45.33% on dry weight basis (39.45%, adb), was higher ash than other biomass feedstocks except for the Chlorella vulgaris (Agrawal & Chakraborty, 2013). Because the inorganic element of algal biomass composed ash content, which was mostly influenced by their environmental culturing, the same algae species might vary in their ash content. This phenomenon could be observed in the case of Chlorella vulgaris as shown in Table 4. Therefore, the ash content of Isochrysis galbana may achieve lower levéis by the appropriate culture condition. A more detailed insight into ash content of Isochrysis galbana in relation to its culturing condition is necessary to be observed. This phenomenon will be revealed in future studies.
The higher heating valué of Isochrysis galbana, which denoted the máximum quantity of energy possibly recoverable from a given its biomass, was comparable to both Cotton residue and Nannochloropsis oculata (Table 4). Moreover, this valué was higher than bagasse. This valué provided the strengthening of the Isochrysis galbana to be prospective feedstock of solid fuel.
The elemental composition of Isochrysis galbana, compared with Nannochloropsis oculata and Nannochloropsis sp., as well as with both Cystoseira tamariscifolia and Bifurcaría bifurcata, was shown in Figure 3. The carbón and oxygen content of Isochrysis galbana, which was expressed in (wt.%) were (14.43±3.25) and (45.68il.ll), respectively. The error indicated the relative standard error of mean from three replication measurements of the sample. These compositions would strongly influence the biomass combustión behavior and the residual characteristics. As a result of the thermal attack on the organically bounds of substance in the biomass, most of the oxygen would be released and then supplied partially oxygen which was required for the combustión processes. In the completed combustión mode, carbón would be burned and accompanied by releasing the amount of heat to form CO2 subsequently.
The inorganic elements presented in Isochrysis galbana comprised: (1) major (>1%); (2) minor (1-0.1%); and (3) trace (<0.1%) elements; in relation to the elemental concentrations. Its major elements and their approximate composition in (wt.%) were Mg (27.98il.57), Ca (5.87Í3.97), Si (1.67±0.40), Cl (1.66±0.52) and Na (1.10±0.26). The minor elements were P (0.88 ±0.45) and S (0.65±0.35) while the only one trace element was found, namely Al (0.07± 0.07). It was already mentioned that the error indicated the same measurement condition of each sample with both carbón and oxygen.
The inorganic elements composition of algal biomass depended on many factors including the type of species, growing processes and conditions, the age of biomass culturing, fertilizer, harvesting time, collection technique, transport and storage mode, pollution, and processing. These characteristics are more changeable and significantly different in comparison among algal biomass depending on aforementioned factors. Figure 3 shows the differences of inorganic elements composition of Isochrysis galbana (eight-day cultivation in Erlenmeyer flask), Nannochloropsis oculata (seventh-day cultivation in the traditionally open pond) and Nannochloropsis sp. (unknown for its culturing condition). Two different species of brown macroalgae, Cystoseira tamariscifolia and Bifurcaría bifurcata that both originated from the same growing environment, season and harvesting technique (Ainane, Abourriche, & Kabbaj, 2015), also had a different composition. Henee, Figure 3 was asserted that the elemental variations among the three species of microalgae, as well as the difference between both species of macroalgae, were depending on various factors, included the culture conditions and type of species. Thereby identify the details of the elemental composition of each raw material, which originated from different species and culture médium, was very critical because it would highly impact to its characteristics in the furnace.
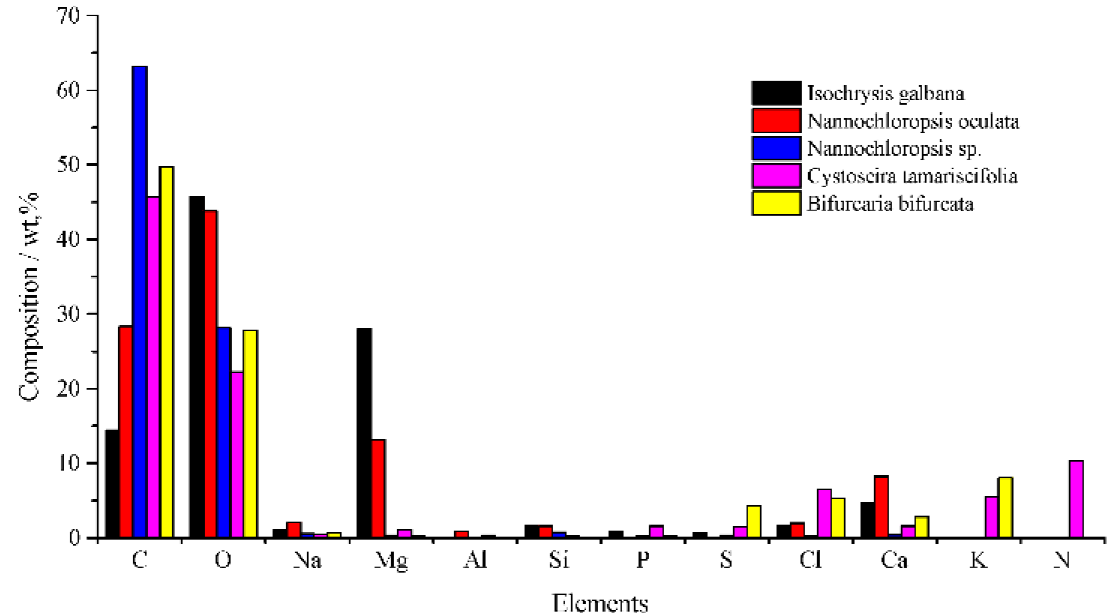
Fig. 3 The EDX elemental comparision of microalgae (Isochrysis galbana, Nannochloropsis oculata (Sukarni et al., 2014) and Nannochlropsis sp. (Patil et al., 2011) and Brown macroalgae (Cystoseira tamariscifolia and Bifurcaria bifurcata (Ainane et al., 2015).
During the combustión processes, inorganic elements could significantly influence the ash melting and deposit formation, aerosol and fly ash emissions as well as the material corrosión and the byproduct utilization of ashes. The respective highest inorganic elements contents of Isochrysis galbana biomass, i.e., alkaline earth metal (Mg and Ca), would frequently increase the ash melting point. The presence of Al in the form of its oxide might also potentially increase the ash melting point, as well. Conversely, the attendance of chlorides and the low melting alkali such as sodium might considerably decrease the ash melting point. Besides chlorides and sodium, the existence of phosphorus could also greatly reduce the melting point of ash (Du, Yang, Qian, Wang, & Chen. 2014; Obernberger, Brunner, & Barnthaler, 2006).
A lot of alkali metáis and chlorides was vaporized during combustión processes to form gasses such as HC1 (g), Na2S04(g) and NaCl (g). Since the heat exchange section of boilers cooled down, they condensed and formed a large amount of fine ash portion. The integration of Cl in the ash was influenced primarily by the concentration of alkali and earth-alkali metáis as well as Si in the fuel, which they could react with Cl. The main effect of Cl in the biomass was the corrosive effect of chloride salts.
The sulfur (S) presented in the biomass forms mostly gaseous SO2, partly also SO3, and both alkali and earth-alkali sulfates. Owing to the succeeding cooling of flue gas in the boiler section of combustión plant, SOx could form sulfates and condensed on the heat exchanger surfaces, or formed fine fly ash particles, or reacted directly with fly ash particles deposited on heat exchanger surfaces by sulphation reaction. The remaining S rests in the flue gas as aerosols and in gaseous formed as SO2 (in insignificant amounts as SO3) (Obernberger et al., 2006). Overall, in-depth knowledge of the inorganic elements composition of algal biomass is critical regarding the technological countermeasures which include proper temperature control on the grate and in the furnace as well as in the boiler section.
3.3 THERMAL CHARACTERISTIC
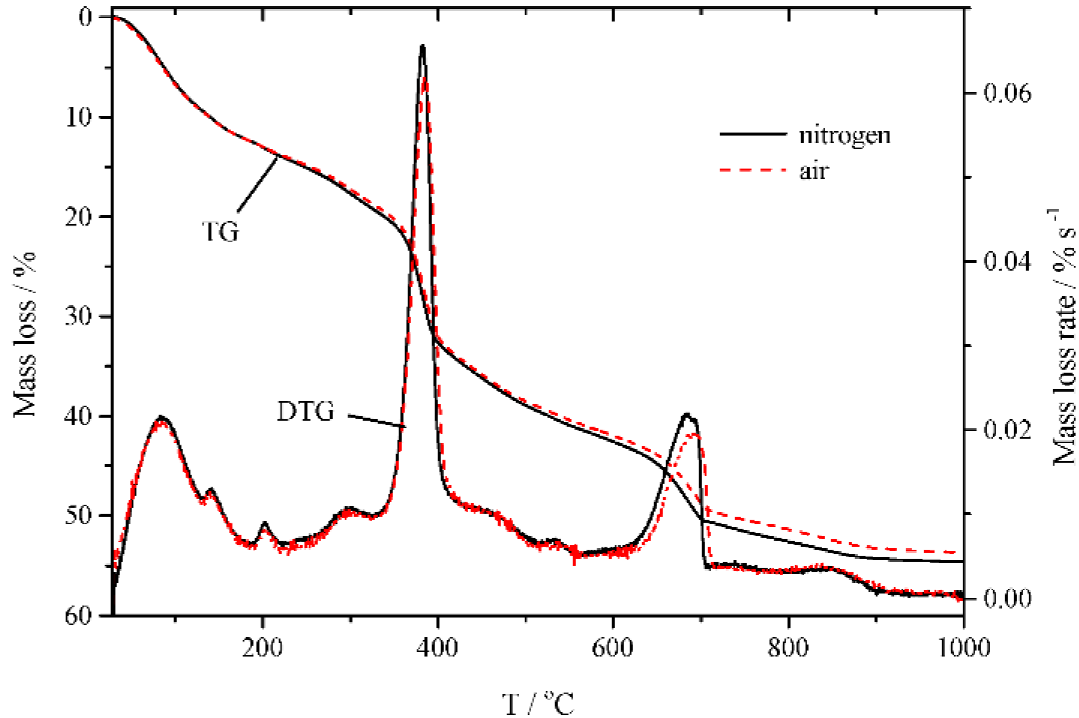
Figure 4 shows the mass loss and the mass loss rate curve obtained during the thermal processes of Isochrysis galbana sample at a heating rate of 10 °C mim1 under nitrogen and air atmosphere, respectively. The both graph has the similar trend, indicated the thermal degradation of the sample was less affected by the kind of the flowing gas. Nevertheless, the presence of N2 has caused the increasing in material degradation at high temperature as compared to that of 02. Figure 4 also signified that onset volatile degradation temperature remains same, but the burnout temperature has been shifted toward higher temperature because of the presence of O2.
Figure 4 revealed that three prominent peaks could be encountered during the thermal processes of the samples. The first stage, which taken place from ambient temperature to nearby 180 °C, was the mass loss relates to the reléase of physically adsorbed water and low molecular weight hydrocarbons. The second stage which characterized by a strong peak started at around 330 °C and finished at about 430 °C attributed to the main pyrolysis and combustión stage. The third stage was the zone where the solid residue of highly non-volatile carbón compounds resulted from stage two gasified and combusted at high temperature.
3.4 FTIR ANALYSIS OF ISOCHRYSIS GALBANA BIOMASS
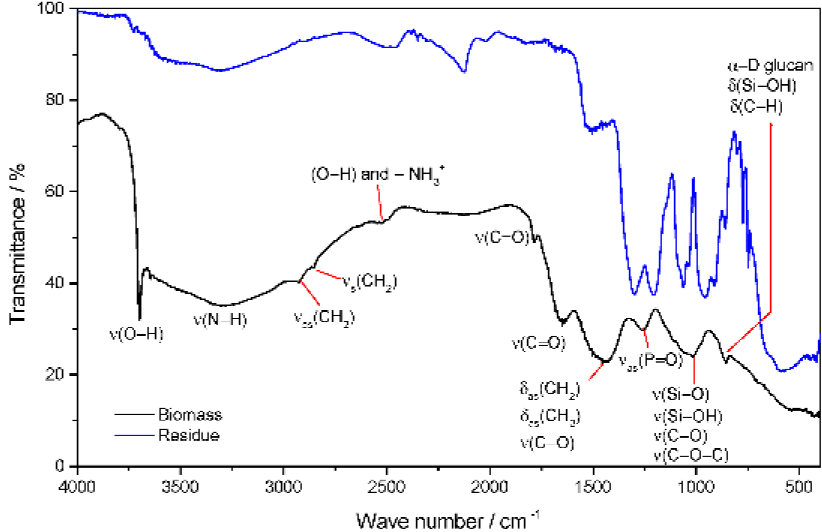
The FTIR spectroscopy provides information on the chemical structure of Isochrysis galbana biomass by identifying the peaks of its spectra, which are produced by the vibrations of functional groups. The FTIR spectra of Isochrysis galbana biomass and its residue 1000 °C were depicted in Figure 5. The band spectra in Figure 5 indicated that Isochrysis galbana biomass contains a number of atomic groups and structures. Band intensities reveal the chemical bonds in Isochrysis galbana biomass which is comprised into three groups: (1) The "carbohydrate band spectra" (2) The "protein band spectra" and (3) the "lipid band spectra"; in relation to the distinct transmittance in different frequency regions.
Peaks in the región where they are supposed to be carbohydrate belonging are referred to the "carbohydrate band spectra." These spectra were distinguished by the weak band at 856 cm1 which indicated the a-D glucans and C-H aromatic deformation of polysaccharides of the cell wall. Likewise, this weak band pointed out the Si-OH bending of silanol (Corradi da Silva et al., 2008; Huang, Jin, Zhang, Cheung, & Kennedy, 2007; Lecellier et al., 2014; Marshall, Javaux, Knoll, & Walter, 2005; Parida, Dash, Patel, & Mishra, 2006; Peng, Zhang, Zeng, & Kennedy, 2005; Peng, Zhang, Zeng, & Xu, 2003; Tianqi, Hanxiang, Manyi, & Tianwei, 2007). The prominent feature at 1010 cnr1 contributing to C-O and C-O-C stretching of polysaccharides, Si-O stretching of siloxanes silicate frustules, as well as its features indicated the Si-OH bond stretching of shanes (Banyay, Sarkar, & Gráslund, 2003; Benning, Phoenix, Yee, & Konhauser, 2004; Benning, Phoenix, Yee, k Tobin, 2004; Dean, Nicholson, k Sigee, 2008; Dean, Sigee, Estrada, k Pittman, 2010; El-Toni et al, 2012; Goo et al, 2013; Guo et al., 2013; Jiang, Yoshida, k Quigg, 2012; Mayers. Flynn, k Shields, 2013; Meng, Yao, Xue, k Yang, 2014; Parida et al, 2006; Stehfest, Toepel, k Wilhelm, 2005).
The "protein band spectra" was shown by the médium peak at 1255 cm1 associated with asymmetric stretching of nucleic acids phosphodiester (P=0) (Banyay et al., 2003; Benning, Phoenix, Yee, k Konhauser, 2004: Benning, Phoenix, Yee, k Tobin, 2004; Dean et al, 2008, 2010; Duygu et al., 2012; Jangir, Charak, Mehrotra, k Kundu, 2011; Mayers et al., 2013; Mecozzi, Pietroletti, k Di Mentó, 2007; Murdock k Wetzel, 2009; Sigee, Dean, Levado, k Tobin, 2002). The most prominent spectrum at 1454 cm1 originated from the CH2 and CH3 bending of methyl and this feature also specified the C-O stretching of the carboxylic group (Banyay et al., 2003; Benning, Phoenix, Yee, k Konhauser, 2004; Benning, Phoenix, Yee, k Tobin, 2004; Dean et al., 2008; Jiang et al., 2012: Murdock k Wetzel, 2009; Sigee et al., 2002; Stehfest et al., 2005). The peak in the spectrum originating from C=0 stretching of esters was the médium band at 1651 cm1, which was associated with the unique protein amide I characteristic (Benning, Phoenix, Yee, k Konhauser. 2004; Benning, Phoenix, Yee, k Tobin, 2004; Dean et al, 2008, 2010; Gao, Yang, k Wang, 2013; Jiang et al, 2012; Kamnev, Ristic, Antonyuka, Chernyshev, k Ignatov. 1997; Mayers et al, 2013; Murdock k Wetzel, 2009: Phukan et al, 2011; Ragusa et al., 2002; Sigee et al., 2002: Stehfest et al, 2005; Wysokowski et al, 2013). The small feature at 1786 cm1 as a result of C=0 stretching of esters (Gao et al, 2013; Jiang et al., 2012; Mayers et al, 2013: Mecozzi et al, 2007; Murdock k Wetzel, 2009; Phukan et al., 2011; Stehfest et al., 2005; Tan, Balasubramanian. Das, Obbard, k Chew, 2013), and less pronounced band at 2520 cm1 owing to superimposed O-H and amine salts -NH3 + stretching (Silverstein, Webster, k Kiemle, 2005) were also invented to be protein band spectra. The broad spectrum at around 3296 cm1 resulted from N-H stretching of amide A has clearly represented the protein of Isochrysis galbana biomass.
The presence of lipid constituent was confirmed by the appearance of two weak peaks at 2856 cm1 and 2927 cm1 related to both symmetric and asymmetric CH2 stretching of methylene (Jiang et al., 2012; Marshall et al 2005; Mayers et al., 2013; Phukan et al., 2011; Sigee et al , respectively. The weak peak at 1786 cm1 originated from C=0 stretching of esters was also supposed to be lipid structure belonging.
These overall FTIR bands spectra have justified the existence of three primary constituents in the Isochrysis galbana biomass, i.e. carbohydrate, protein, and lipid. These constituents were the primary substances, which composed several microalgae cell structures. The algal cell wall was composed of a polymer of 1,4 linked β - D - glucose and an amorphous mucilaginous material constituted of polysaccharides, lipids, and proteins. The plasma membrane was composed of phospholipid bilayer and protein membrane. The chloroplast consisted of several membranes composed of lipid and protein, and it contains small spherical lipid droplets between the thylakoids. The mitochondrion's inner membrane contained of proteins greater than 70%. All cell structure constituents could be gradually cracked in the course of the thermal processes when the temperature extended to its decomposition temperature, and the fraction of material would be released to be volatile matter, while the residual constituent formed char as a solid material. Both volatile and char would be combusted during the combustión processes. The latest leftover material after the final combustión processes was ash as the last remaining material. This ash material was mainly arranged from the inorganic ingredient of biomass. The overall thermal decomposition phenomenon have been shown by progressive declining intensity in the spectra of biomass residue 1000 °C, mainly at around 3590 -1630 cm-1.
4. CONCLUSIONS
The several significant characteristics of microalgae Isochrysis galbana for future solid biofuel have been investigated. In the growth kinetics point of view, with simple and inexpensive nutrient culturing, this alga has comparable growth rate to other algal species. The Isochrysis galbana physical properties indícate its visibility as compared to both aquatic biomass and the terrestrial plant. The elemental analysis of Isochrysis galbana points out that this biomass has a proportionality valué against other algal biomass. The particular attention must be given to the high Mg content of this biomass since it could affect the residual behavior in the combustión system. The macromolecular contents of this alga has also indicated the presence of three primary constituents inside its biomass in terms of carbohydrate, protein, and lipid, which they thermally degraded and then converted into energy during the combustión process. The overall biomass characteristics as already mentioned above provides the essential information regarding the prospect of this alga sustainable solid biofuel feedstock and offers Isochrysis galbana as a possible candidature for future bioenergy source.











 nueva página del texto (beta)
nueva página del texto (beta)