1. Introduction
Ozonation as pre-treatment of textile dye wastewater is an efficient step for improving wastewater biodegradability, as well as reducing acute ecotoxicity, which can be removed completely through sequential biological treatment (Somensi, Simionatto, Bertoli, Wisniewski, & Radetski, 2010). Abidin, Fahmi, Soon-An, Makhtar, and Rahmat (2015) reported that the application of ozonation as pre-treatment for biological treatment may further mineralize the dye-containing wastewater. COD was reduced simultaneously by ozonation and biological treatment mechanism at lower ozone doses (Abidin & Ridwan, 2011). Punzi et al. (2015) used anaerobic biofilm reactor followed by ozonation for treatment of textile wastewater containing azo dyes. In combined treatment, ozonation and biological method in wastewater, ozone removed COD, color and pathogens and increased the biodegradability of the wastewater (De Souza, Bonilla, & De Souza, 2010).
A new combined treatment process, ozonation and anaerobic biodegradation by UASB reactor has been proposed to see the degradation effect of dye wastewater, so that an efficient and economical, wastewater treatment system could be generated.
2. Materials and methods
Reactive Black 5 as di-azo dye synthetic solution was used for making synthetic wastewater. The concentration and pH of dye wastewater were very high i.e. 1500 mg/L and 10.13. Figure 1 shows the chemical structure of Reactive Black 5. Ozone was generated by corona discharge type ozone generator model Eltech el-5g/h.-A with flow rate of 5 g/h. Ozonation of dye solution was carried out in a batch mode. Detail ozonation procedure including decomposition mechanism and experimental analyses have been described in earlier work (Venkatesh, Quaff, Venkatesh, & Pandey, 2014).
The biological analysis was performed in a lab scale upflow anaerobic sludge blanket (UASB) reactor shown in Figure 2. The biomass in the UASB reactor was conditioned sludge. This conditioned sludge was prepared using fresh sludge fed with synthetic media containing 500 mg/L sucrose for 30 days in a laboratory. The fresh sludge was obtained from the anaerobic digester tank of full-scale activated sludge process based sewage treatment plant at Bakshibandh, Allahabad, India. Reactor was fed by synthetic wastewater containing sucrose as carbon source whose COD was 534 mg/L up to steady state condition achieved. The flow rate was 25 mL/h, which translated to up flow velocity 0.16 m/h and hydraulic retention time (HRT) of 40 h. Yasar and Tabinda (2010) reported UASB reactor performs better removal efficiency at lower hydraulic retention time. After achieving a steady state condition, the reactor was continuously running on synthetic medium strength domestic wastewater. After that, the reactor was subjected to ozonated synthetic dyes solutions. The ozonated azo dye solutions were mixed with synthetic wastewater in a 1:1 ratio. For determination of the extent of anaerobic biodegradation, mixed ozonated azo dye solutions were used as feed for anaerobic bacteria and assessed for the extent of biodegradability in UASB reactor.
3. Results and discussion
3.1. Effect of ozonation on dye wastewater
Decline in pH value of Reactive Black 5 dye samples were observed during ozonation. This decreases rapidly from 10.13 to 3.30 in 25 min whereas conductivity of dyes solution increased during ozonation. Increase in conductivity after ozonation may partly be attributed to an indirect confirmation of ion accumulation. Figure 3 displays the variation in pH and conductivity at 25°C.

Fig. 3 Variation in pH and conductivity at 25°C with ozonation time for initial dye concentration of 1500 mg/L of Reactive Black 5.
After 25 min of ozonation the initial concentration of dye wastewater reduced from 1500 mg/L to 97.4 mg/L. The degradation of high concentration Reactive Black 5 dye molecules required longer ozonation time. Ozonation of dye solution reduced the COD concentration. 50% COD reduction occurred in 10 min of ozonation time, although in some cases the values of COD increase with ozonation time. An increase in COD value was mainly because of an organic species produced due to destruction of the molecular structure of azo dye by ozone (Constapel, Schellentriager, Marzinkowski, & Gab, 2009; Venkatesh, Quaff, Venkatesh, & Pandey, 2015). Figure 4 presents the dye concentration and COD decline during ozonation of Reactive Black 5 dye solution. Fahmi, Abidin, and Rahmat (2011) reported increases of COD were observed during ozonation process due to dye molecules being oxidized resulting in formation of small organic molecular fragments, such as acetic acid, aldehydes, ketones, which are not completely mineralized under the oxidative conditions, contributing to the increase in COD.
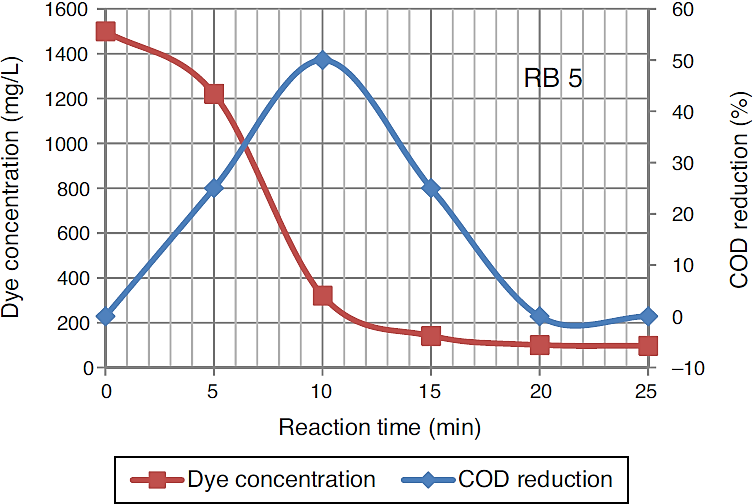
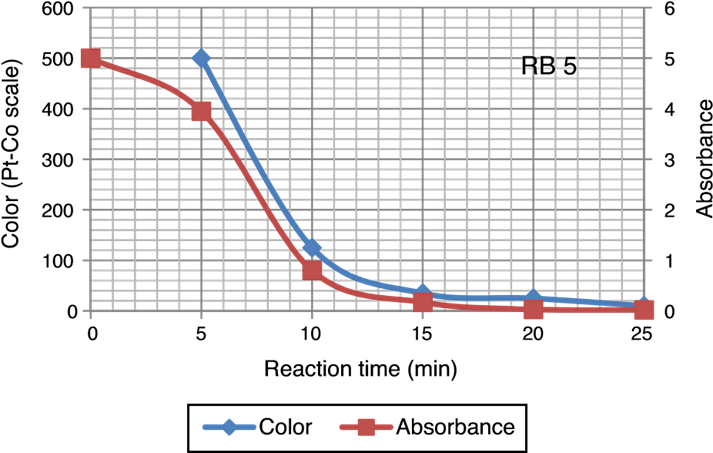
Fig. 4 Reduction in the dye concentration and COD during ozonation of Reactive Black 5 dye solution.
Color of dye solution was declining exponentially with increase ozonation time. It achieved around 70% decolourization in 10 min. Decolourization efficiency was 94% after 25 min of ozonation investigated in this study. Ozonation treatment leads to a complete decolourization of the Acid Black 1 dye solution in 25 min (Paprocki, Dos Santos, Hammerschitt, Pires, & Azevedo, 2010). A rapid decline in color (Pt-Co scale) and absorbance shows that the decolouration dynamics in Figure 5. In Reactive Black 5 azo dye solution, ozonation resulted in progressive increases in biodegradability ratio. It increased from 0 to 0.4 after 25 min of ozonation. The extent of mineralization of dye molecules can be explained by the reduction in TOC content due to ozonation. During ozonation, the dye molecules being oxidize and mineralize by attack of ozone on aromatic rings and unsaturated sites on dye molecules. The TOC of azo dye solutions before and after ozonation was 173.94 and 132.2 mg/L respectively. Other researchers (Gharbani, Tabatabaii, & Mehrizad, 2008; Tehrani-Bagha, Mahmoodi, & Menger, 2010) have also observed similar results in reduction of TOC by ozonation of dye solutions. Sundrarajan, Vishnu, and Joseph (2007) reported 50% COD and 40% TOC reduction were achieved by ozonation from a dye bath effluent containing several conventional reactive dyes of different shades.
3.2. Mineralization of ozonated azo dye solutions in UASB reactor
To determine the extent of anaerobic biodegradation by anaerobic bacteria in UASB reactor, the dye solutions after ozonation were mixed with synthetic wastewater in a 1:1 ratio and used as feed for anaerobic bacteria in UASB reactor. The results demonstrated that the UASB reactor performance varied from 60 to 83% for COD reduction. The reactor was operated at low organic loading rate 0.1-0.2kgCOD/m3 d. The influent COD was 478.0 ± 3.00 mg/L. No significant variations in the effluent characteristics were observed. The COD of the UASB effluent was 40.29 ± 2.46 mg/L. Changes in pH and alkalinity were insignificant. Ganesh, Ramasamy, Gajalakshmi, Sanjeevi, and Abbasi (2007) observed the UASB reactors achieved total organic removal efficiency of 75-85%.
3.3. Analysis of removal of organic compounds by combined process
The degradation of azo dye was monitored, by the study of the absorbance of the untreated and treated dye sample in UV absorbance range from 200 to 1000 nm. Figure 6 shows the UV-vis spectrum variations of Reactive Black 5 dye solutions. Results indicate that the dye is destroyed by ozone very rapidly. The specified wavelength peak (λmax 600 nm) has been found to disappear and a continuous decrease in absorbance has been observed. From results, it signifies that the ozone-oxidation can remove the organics present in the dye wastewater samples. When ozonated dye solution were further treated through anaerobic process, it is observed that the absorbance at those wavelength is less compared to the absorbance after ozoneoxidation stage, which signify that the remaining organics are also being removed by anaerobic process. Fahmi, Abidin, and Rahmat (2010) observed ozonation transforms the functional groups in azo dye to produce more biodegradable by products, which are easily removed by biological treatment.
3.4. By-products identification by ion chromatography
The products formation after combined treatment of azo dye solutions were identified by ion chromatography and are presented in Table 1. Chromatogram of this analysis is presented in Figure 7. These ions found after ozone treatment were also present after anaerobic biodegradation in very low concentrations. Anaerobic biodegradation in UASB reactor resulted in complete mineralization of oxalate ions. During ozonation, the cleavage of the dye results in aromatic amines and their further oxidation/ozonation leads to organic acids. Further ozonation resulting oxidation and cleavage of amine groups (NH2) in the original dye molecules were oxidized to nitrate. Oxidation and cleavage of sulphonic groups result in the formation of sulphate observed in this study.
Table 1 Analysis of by-products of Reactive Black 5 dye solution.
| Inorganic/organic ions concentration (mg/L) | After ozonation | After anaerobic biodegradation |
| Sulphate | 134.11 | 55.22 |
| Nitrate | 19.63 | 18.48 |
| Nitrite | ND | 0.26 |
| Fluoride | 1.2 | 0.48 |
| Chloride | 525.93 | 266.83 |
| Phosphate | ND | 15.16 |
| Oxalate | 107.53 | Nil |
The degradation mechanism of ozonation in dye molecules, leading to the release of benzene and naphthalene derivatives as ozonation by-products which further oxidize in aldehydes, ketones and other aliphatic compounds was observed by Zhang, Yediler, and Liang (2007) and Zhu (2010). Ozonation of azo dye solutions resulted in formation of aromatic and aliphatic compounds, which are further degraded by anaerobic biodegradation and shows enhanced BOD5/COD ratio.
The decomposition of ozone in water is the chain mechanism which is combined with initiation, propagation, and termination. The oxidation occurs through highly reactive hydroxyl radicals. These free radicals come from reaction mechanism. Such free radicals are readily available to react instantly with organic compounds such as dyes (Alvarez, Pocostales, & Beltran, 2011; Siles, García-García, Martín, & Martín, 2011). Buehler, Staehelin, and Hoigne (1984) proved experimentally that the hydroxyl radicals are dominant decomposition products of ozone in aqueous solutions, and accelerate the decomposition of ozone. Ozone reaction is more inclined to direct reaction with increasing the inhibitor concentration (Guo, Yang, Cheng, & Wang, 2012).
A simple mechanism for the decomposition of ozone in aqueous solution is illustrated in following Eq. (Selcuk, 2005).
Hoigne, Staehelin and Bader (HSB) model, has described the decomposition process of ozone in aqueous solution. The decomposition routes of ozone with reaction rate constants are following equation (Staehelin & Hoigne, 1985; Langlais, Reckhow, & Brink, 1991):
In the present study, the absorbance decreases (Fig. 5) in the UV region, which indicates the formation of new compounds or a breaking of these molecules into simpler ones. In the present study, the increase of the dyes solution conductivity after ozonation may serve as an indirect confirmation of sulphate and nitrate ions accumulation. On the other hand, sulphate, nitrate and oxalate ions were identified as final accumulated products and such compounds are classified as the final reaction products. Perez, Poznyak, and Chairez (2013) reported final degradation products of ozonation of azo dyes have been identified to be sulphate, nitrate, formate and oxalate.
3.5. Observation on combined process for the treatment of azo dye wastewater
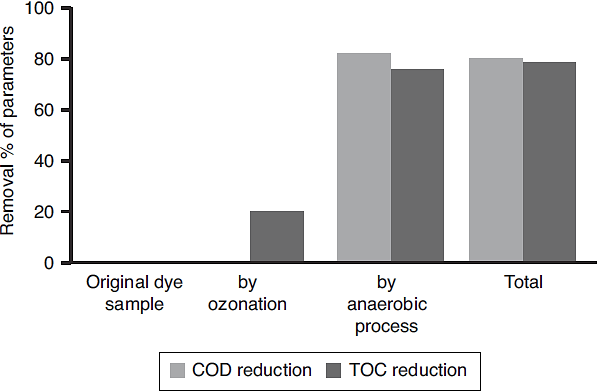
The results showed that the combined process in which ozonation and anaerobic biodegradation play a major role in reduction of color and COD of the dye wastewater. It has also observed that longer ozonation time is required to degradation of high concentration of Reactive Black 5 dye molecules. This is immensely affecting the process cost. Marco, Esplugas, and Saum (1997) reported that total mineralization through oxidation process is highly expensive whereas a combination of the oxidation process and biological option would be a cheaper method for degradation of total organics. Thus combined treatment to reduce the cost of the wastewater treatment process by ozonation, anaerobic treatment process by UASB reactor was used along with ozonation in this study. Ozonation of landfill leachate was proven to as the pre-treatment enhance biological treatment efficiency with total removal rate was 71.94% (Qiao, 2012). Combined treatment reduced the COD level much more significantly, which is about 90%. It also reduces TOC remarkably shown in Figure 8. The color showed less than 10 on Pt-Co scale has shown in Figure 9. Table 2 shows organic contents removal after combined process. Ozonation as a pre-treatment for combined chemicalbiological treatment is a potential process for enhanced color removal efficiencies greater than 96% were obtained (De Souza et al., 2010). Wang, Chen, Gu, Wang, and Qian (2008) reported aerated filter biological process as post-treatment after ozonation were used to treat textile-washing wastewater. The result showed that the influent qualities were COD about 80 mg/L and color 16 degree, after combined process the effluent qualities of COD less than 30 mg/L and color 2 degree respectively.
Table 2 Organic contents removal after combined process.
| Parameters | Original dye solution | After 25 min ozonation process | Influent for UASB (ozonated dye solution & synthetic domestic w/w in 1:1 ratio) | Effluent of UASB reactor | Overall % removal |
| Reactive Black 5a | |||||
| pH at 25°C | 10.13 | 3.3 | 7.52 ± 0.045 | 8.49 ± 0.411 | 90 |
| COD (mg/L) | 426.66 ± 0.54 | 426.66 ± 0.36 | 478.0 ± 3.00 | 40.29 ± 2.46 | - |
| BOD (mg/L) | ND | 173 | 193 | 18 | 84 |
| TOC (mg/L) | 173.94 | 132.2 | 149 | 27.63 | - |
| BOD5/COD ratio | ND | 0.41 | 0.41 | 0.42 | - |
| Color (Pt-Co scale) | ND | 10 | BDL | 10 | - |
| COD/TOC ratio | 2.45 | 3.22 | 3.22 | 1.54 | - |
| Conductivity (mS/cm) at 25°C | 1.935 | 2.35 | 1.2 | 1.1 | - |
a Dye concentration: 1500 mg/L.
4. Conclusions
A new combined and economic process was developed using ozonation and subsequent anaerobic process for treatment of dye wastewater. This combined process achieved 90% of total COD and 84% of total TOC reduction. It was also observed that for reduction of high COD, color and TOC required, longer ozonation time which affected the entire cost of the treatment. Thus anaerobic biodegradation by UASB reactor have been used along with ozonation to reduce the COD and TOC level as well as to optimize the ozonation treatment process. The operational parameters like pH, conductivity, color and total organic carbon (TOC) were also analyzed and results showed that the initial pH value decreased with ozonation contact time, which indicated that the generation of by-products with acidic nature (inorganic anions and organic acids) as a result of oxidation by ozone. The dye concentration decreases with increases in ozonation time. Ozonation treatment leads to a dye removal up to 94% of the initial concentration in few minutes (<25 min) and color removal showed less than 10 on Pt-Co scale. Ozonation resulted enhancement of BOD5/COD ratio of the dye solutions. The ozonized dye solution showed the presence of organic anion like oxalate ions and inorganic anions (sulphates and nitrates) as final degradation by-products.











 nueva página del texto (beta)
nueva página del texto (beta)