Introduction
Progressive multifocal leukoencephalopathy (PML) is an infectious and opportunistic disease caused by the John Cunningham virus (JCV), which attacks oligodendrocytes and astrocytes, causing demyelination of the central nervous system (CNS) and significant disability in the patient1.
The JCV, isolated for the 1st time in 1971, is a polyomavirus that belongs to the Polyomaviridae family and is composed of Deoxyribonucleic acid2,3. Contact and primary infection occurs in childhood, it is typically asymptomatic, and the virus remains latent in the kidneys and lymphoid organs4. However, in an immunosuppression state, JCV can be reactivated5.
In patients with recurrent remitting multiple sclerosis (RRMS), an autoimmune, inflammatory, and demyelinating disease of the CNS, the immunosuppressants or immunoregulators are administered as disease-modifying treatment. One of them is natalizumab, a monoclonal antibody that binds to the α4β1 integrin on the surface of autoreactive lymphocytes, preventing its binding with endothelial VCAM-1 and its passage through the blood-brain barrier6. Due to its efficacy, this drug is widely used throughout the world. Unfortunately, it has been associated with the development of PML. At present, three risk factors have been proposed for developing an infection in RRMS patients treated with natalizumab: (1) level of anti-JC virus antibodies (JCVAb); (2) more than 2 years of treatment; and (3) previous history of immunosuppressive therapies7-9.
The quantification of JCVAb is of particular interest since it has been directly proportionally associated with the development of PML10. Reports of seropositivity prevalence for JCVAb in different countries were between 30% and 90%11,12. In Spain, a multicenter study was carried out with a large sample, finding a prevalence of seropositivity of 55.3% without identifying differences with other regions studied at that time13. Another study in Poland reported a prevalence of 63.1%, one of the highest in Europe14. While in Korea, the prevalence was up to 80%15, and in Kuwait, it was low, falling at the lower limit of the ranges established worldwide16. About Latin America (LATAM), a systematic search identified a JCVAb seroprevalence study conducted in Brazil in 2013, being the only study from LATAM17. No publication was identified in Mexico.
This study objective was to determine the prevalence of JCVAb seropositivity in a sample of patients with MS in Mexico and their behavior of seroconversion.
Methods
The Instituto Mexicano del Seguro social Ethics Committee approved the study (R-2017-3601-14); all data were obtained from the clinical record and handled according to the privacy laws of personal data. An observational and retrospective study conducted at the Hospital Centro Médico Nacional Siglo XXI in Mexico City, a highly specialized center, where were included patients with RRMS (McDonald criteria 2017)18, older than 18 years, who had at least one serum JCVAb determination, from the period of November 2015 to November 2020.
Serum samples were taken before the start of treatment with natalizumab and continued to be performed periodically, on average, every 6-12 months in patients who merited and accepted it. The antibody index was determined using the STRATIFY JCV ™ test, enzyme-linked immunosorbent assay (ELISA) in a reference laboratory, Quest Diagnostics Infectious Disease, Inc., California19. The test consists of a two-step ELISA and a complimentary confirmatory test.
The JCVAb result was expressed as an antibody index (≤ 0.2 was considered negative, ≥ 0.4 positive and between 0.21 and 0.39 indeterminate). The samples with indeterminate levels were subjected to a confirmatory test by the Quest Diagnostics Infectious Disease laboratory. Based on the publications of risk staging, the JCV index measurement was considered according to their level of risk of developing PML as next: ≤ 0.9 low, 0.91 to 1.49 moderate, and ≥ 1.5 high20.
Statistical analysis
We used the SPSS version 25 program for Mac. Seroprevalence was reported as percentage. Qualitative variables were reported as frequencies and percentages; quantitative variables were expressed as mean and standard deviation. For the bivariate analysis, the Shapiro-Wilk test was used to evaluate the normal distribution, being an abnormal distribution. Therefore, the data were analyzed using non-parametric tests; Chi-square was used for qualitative variables. Linear regression analysis was also performed to evaluate the influence of JCVAb index and variables; p ≤ 0.05 was defined as statistical significance.
Results
A total of 93 patients with RRMS who had at least one JCVAb determination were included from November 2015 to November 2020. It was also analyzed whether there was a relationship between the high index and the patient's age without finding a statistically significant relationship.
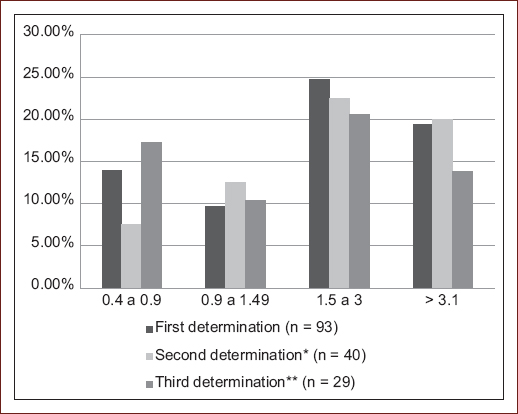
We studied seroconversion in a subgroup of 40 patients who had at least two measurements of anti-JCVAb antibodies. The average index of the second determination was 1.45 ± 1.33 (min 0.05 - max 4.19). About 87.5% (n = 35) remained stable in their serological status, with an average follow-up duration of 25.3 ± 13.87 months. In this subgroup, 15 patients were in a seronegative or indeterminate state, of which 12 (80%) remained without changes in their status, and 20% (3) seroconverted to positive in a mean time 15.2 ± 9.6 months. Of the seropositive patients in the initial determination, only 2 (5%) changed to an indeterminate state, and none reached a negative conversion. The third determination was carried out in 29 patients, with an average index of 1.26 ± 1.20 (min 0.06-max 3.59) figure 1. Only one patient presented seroconversion from positive to indeterminate. We did not identify statistically significant factors that influenced the conversion to positives such as age or gender compared to the group that remained negative.
Discussion
In our study, we identified a prevalence of JCVAb seropositivity (≥ 0.4) of 67.7%, which is positioned as one of the highest reported in various studies that range from 40.6 to 69% according to the country21. However, other studies place this general prevalence between 30% and 90%22. At present, several regional or multicenter studies have measured the prevalence of positive JCVAb. However, their comparison may generate bias because some have methodological differences in the cutoff points to define positivity and the number of patients. Even so, we consider it worthwhile to determine the differences between these studies and our results. We identified countries with JCVAb prevalence in the lower range, such as Australia 48.6%, UK 48.8%12,21, Kuwait 44.2%21,23, and Norway 47.4%11,21. While the countries that share similar JCVAb prevalence to Mexico were: Austria 66.7%11,12, Portugal 69.5%, Belgium 66.7%12, Turkey 67.7%11, and Korea up to 80%, although the vast majority countries are between 55 and 70%15,21.
We do not know the reasons for this variability of results between countries, although it has been proposed to attribute it to different ethnic groups. However, the JEMS multicenter study did not identify a statistically significant difference between the races studied and positive seroprevalence12.
Regarding gender and age, some studies have associated a higher frequency of positivity and a higher JCVAb index in male and older people13,24. However, we did not observe a significant difference in our population concerning gender and age regarding seropositivity, behaving in this respect similar to the Korean population15.
We analyzed patients according to PML risk stratification, finding a higher frequency in the index range between 1.5 and 3. Fortunately, there were no cases of PML in the population studied over 5 years.
Regarding the behavior of the serological status in the longitudinally studied subgroup, 87.5% remained unchanged, similar to the 84.5% reported in the study by Alroughani et al.16. While only 3 (3.2%) seronegative patients changed their status to seropositive, different from the results obtained in Spain with a seroconversion of 7% and in Kuwait of 11.8%13. Thus, our rate of being positive conversion was lower than reported.
In our study, factors such as age and gender do not contribute to seroconversion. It is possible that the switch to seropositivity depends on the immunological status of the patient. This is influenced by different elements such as treatment (for example, natalizumab or fingolimod), comorbidities, and nutrition. By proposing this theory, we understand the importance of extending the measurement of other elements that could influence in the behavior of the VJC status.
Our study has some limitations due to the sample size compared to other studies, especially multicenter studies, because we carried it out in a single center. However, as we do not have much information on the subject in the LATAM population, our study provides one of the few prevalence references in LATAM.
In summary, this study identified a prevalence of JCVAb seropositivity of 67.7%, located in the upper range globally, without finding a relationship between the antibody index and age. The positive seroconversion rate was lower than reported. A multicenter study is required to extrapolate our results.











 nueva página del texto (beta)
nueva página del texto (beta)