Introduction
In recent decades, neuroscientists have shown a particular interest in studying the causal relationship between consciousness and underlying neural activity. The relationship between the nervous system and behavior has generated various questions about how our brain generates various patterns of perception secondary to the stimuli that we receive from the external environment and how the brain’s neural networks process information. Precisely, the purpose of this work is to provide an overview of the neural bases of consciousness and the main neural processing models of it that are in force to this day.
Consciousness could be defined as a physiological state that varies according to the temporal and spatial domain of its neuronal operations, finally allowing the appearance of complex and conscious behaviors1. In this sense, it is also possible to specify that consciousness is not an attributable element exclusively to human beings. In this sense, a series of theoretical postulates that refer to this fact have been offered over time, fundamentally through the concept of “Neural Darwinism” or also known as Theory of Selection of Neural Groups, and a concept that has been widely raised and supported by three methodological assumptions that are set out below2:
− The physicalist assumption, which points out that consciousness is a particular form of physical process that arises from the structure and functioning of certain animal brains.
− The evolutionary assumption, which states that both the structure and the function of the nervous system as well as consciousness are the result of natural selection processes.
− The assumption of qualia, alluding to the qualitative characteristics of conscious experience and denoting the properties of certain mental states3, which are real, internal and private states and to which only the individual himself has access. With this, they cannot be communicated directly by any scientific theory.
The evolution of consciousness according to neural Darwinism
In this sense, Edelman has affirmed that there is neuronal activity underlying the phenomenal experience. Moreover, in this way, he argues that there are two linked concepts: primary consciousness and secondary (or higher order) consciousness. Both are intended to explain the origin and evolution of consciousness.
In the first place, primary consciousness is defined as the ability to establish a mental scene that integrates a large amount of different information with the aim of guiding a present or imminent behavior2 and has the following characteristics:
− It occurs in species that have brain structures similar to humans.
− It occurs in animals that are capable of constructing a specific mental scene, but that lack highly complex semantic or symbolic elements.
− Short-term memory is required for its appearance.
− Individuals who possess it are unable to report their own qualias or those of other subjects.
Second, secondary or higher order consciousness is conceived as the consciousness of the conscious being and is established as a characteristic of human beings2. It is characterized as follows:
− Consider the prior existence of a primary consciousness.
− It is accompanied by a sense of one’s own identity and the ability to construct past and future scenes in the midst of a waking state.
− Unlike primary consciousness, it requires higher order cognitive processing, with greater semantic and symbolic resources.
− It allows to develop the concepts linked to one’s own subjective identity (the self), past and future elements.
In this way, it is possible to appreciate that the concept of consciousness has been coined over time as an element closely related to psychology, philosophy, and even within terms associated with evolutionary biology. Over time the proposals on this concept have been refined and proposing new conceptual bases.
Consciousness as a state and as an experience of a content
In this line, it is possible to appreciate that our brain establishes mechanisms that allow us to be in a certain mental state, constituting itself as an geological theory of consciousness, where the ego or the self-accounts for and is part of a certain content of phenomenally conscious states4. Based on this, it is possible to consider consciousness from two quite clear perspectives: the level of alertness or arousal, where the existence of energy levels or bodily and mental activation that a subject possesses at a given moment is appreciated, and on the other hand the experience of a content or consciousness where the subject is perceiving their own internal states as well as the different stimuli that are present in the environment and that generate an impact on behavior5. With this, our brain always processes an experience that in any case involves the existence of a subject of experience, who is fully aware that he is in a certain mental state. Both elements, both the level of alertness and the experience of a content, are subject to neuronal activity6.
An example of the above is the existence of different states of brain activity that occur under the influence of anesthetics and also in the perceptual mechanisms. Regarding the first point, it is possible to determine that the existence of anesthetic drugs have an important influence on neuronal physiology, such as propofol and entomidate7,8. In this case, the levels of cerebral alertness are considerably reduced. In this regard, interesting techniques for monitoring neuronal activity in this state have been considered, such as electroencephalography (EEG), which allow recording while the subject is under the effect of anesthesia. The signals generated by the application of this procedure have oscillatory characteristics and are analyzed within five frequency bands. This oscillatory activity is usually modified when the state of consciousness changes, where neural networks exhibit a reduction in their beta rhythm and an increase in alpha rhythm9.
It is interesting to note in this regard that, although the subject is in a prolonged state of rest, it is not possible to completely rule out the existence of certain levels of neuronal activity, demonstrated by studies carried out applying functional magnetic resonance imaging in anesthetized subjects10,11. This also shows the emergence of intrinsic brain activity during rest and based on synchronized neuronal activity patterns12. From this point of view, it can be seen that the levels of arousal are largely regulated by neural networks that supply the attentional tone in corticosubcortical areas linked to the right hemisphere, where the anterior cingulate acts as the central coordinator13. On the other hand, the existence of a controller of arousal levels is also indicated, such as the right prefrontal cortex in conjunction with the participation of the anterior cingulate and some frontal medial structures14. This also proposes a neurotransmission network based on discharges of noradrenergic neurons and, to a lesser extent, serotonergic neurons of the nigrostriatal system of the right hemisphere. At the level of the left hemisphere, an important participation of dopaminergic and cholinergic networks (the latter to a lesser extent) has been seen15. In this way, the arousal levels may present various tonic changes linked to signal improvement or noise reduction and also phasic changes, which are linked to variations in information processing and status updating in tune with the type of activity being carried out, which has a direct impact on the subject’s alertness levels. This is where various subcortical areas and neurotransmission systems that regulate the locus coeruleus and noradrenergic networks are also involved16.
We must consider here two important concepts: the persistent vegetative state and the minimally conscious state. The persistent vegetative state implies a clinical situation of total ignorance of oneself and the environment, with the presence of sleep-wake cycles, and complete or partial maintenance of hypothalamic and brainstem autonomic functions. On the other hand, the state of minimal consciousness is a condition, in which patients have cognitively mediated and minimal responses such as following simple instructions, verbal, or gestural yes/no responses, among others17. Based on the above, some studies have also been carried out related to the vegetative state and in people who exhibit a minimal state of consciousness, entities directly related to consciousness understood as a state. A study carried out by Noe et al., in 2019, indicates that people who have been in a state of minimal consciousness have been able to get out of this state and recover within a hospital environment and with important therapeutic support in different axes such as sensory and physical stimulation for at least 2 h daily. This recovery process was much more favorable than for those people who were in a persistent vegetative state. This indicates that both the persistent vegetative state and the minimally conscious state are clinical entities that differ in both diagnosis and prognosis18. In children, some findings have also been seen when they have been affected by a persistent vegetative state. The evidence shows that there is a diffuse decrease in cerebral metabolism thanks to the application of neuroradiological studies19. However, there is little evidence showing the evolution of children affected by persistent vegetative state and/or minimally conscious state, so updated studies are required to contribute to decision-making regarding treatment of the pediatric population suffering from these neurological conditions.
When we refer to consciousness as an experience of content, that is, according to the awareness concept mentioned above, it is conceived as an experience of an individual type and that, therefore, it is not possible to be transferred, although we can interpret and understand it from the behavior20. This element has acquired a particular relevance in recent times, becoming a true representational theory of consciousness. From this point of view, it is possible to define what is known as phenomenal consciousness, which certainly combines the qualities felt or experienced (such as a cloudy sky or a particular aroma) together with the subjective nature of said experience and how the subject appropriates it or also, how the phenomenon impacts on the individual21.
If we delve into this aspect, there is much to say, especially when we refer to the neuronal changes that occur in a conscious process linked to content. If we consider that this is generated in the presence of a stimulus, we can find several neural circuits that participate and with different states of activity:
− When a stimulus arrives through the sense organs, the information passes through the thalamus and reaches the primary sensory cortex after 20 ms after the presentation of said stimulus22.
− If this neuronal activity is intense enough, it will spread to what is known as the “global workspace” (an area made up of distributed and interconnected neurons, with long-range axons and a set of modular perceptual processors, motor activity, memory, evaluation, and attention)23.
− When the above occurs, feedback will be generated from the global workspace to the primary sensory area, 80–100 ms after the stimulus is presented. It is important to highlight that the neuronal activity of the primary sensory cortex must occur in order for the conscious perception of the stimulus to be generated24,25.
− Only if a motor action is required to inform the conscious perception or non-perception of the stimulus, the prefrontal cortex will have a high role, a few milliseconds after receiving the stimulus26,27.
As mentioned, the activity of the primary sensory cortex increases considerably in a conscious process, especially in the indicated feedback process. Corticocortical synapses in the primary sensory cortex generate important local field potentials (LFPs), which are large extracellular electromagnetic fields produced when several presynaptic axon terminals act synchronously by contacting the dendrites of neocortical pyramidal neurons28.
This is where the concern then arises as to why the individual action potentials of the neurons of the primary sensory cortex are not related to conscious processes29,30 and local field potentials do31,32. The answer lies in the fact that the individual action potentials of these neurons are of short duration and do not propagate far from where they are generated, considering that this activity is measured extracellularly. That is why it is perfectly possible to think that conscious experiences can be transient spatial patterns of LFPs or transient spatial patterns of electromagnetism28.
Neural correlates of consciousness
At a general level, it is possible to define this aspect as the set of events that are seen in the human brain when a conscious mental state occurs that can be observed or measured with different brain imaging techniques33.
For many years, different theoretical lines have been presented regarding the study of consciousness. This has led to the development of models that allow the understanding of different mechanisms of neuronal interaction and neurofunctional levels that support the conscious process. In this way, it is possible to consider a series of loops that correspond to this neurofunctional model.
The first of them is made up of the ascending reticular activator system, connected with the nonspecific nuclei of the thalamus34 and also with the hypothalamus35. This network allows the subject to control the physiological activation of the individual and makes the higher brain structures also operate physiologically36. With this, it is possible to appreciate that, to generate states of higher processing of consciousness, it is necessary previously to have structures that serve as a basis for this purpose.
Second, we have another neuronal loop, made up of the thalamus and its main structures, which are the non-specific nuclei of the thalamus and the neuronal connections that they maintain with the entire cerebral cortex and also with subcortical areas. These connections are bidirectional in nature, maintaining important levels of interrelation between the different structures that comprise it37. In this way, this neural network causes an important distribution of information to be generated in cortical and subcortical areas, increasing the neural substrate for it. There is also a network made up of a thalamic nuclear axis composed of a thalamic reticular nucleus, the intralaminar nuclei, and the nuclei of the midline of the thalamus37 and which is responsible for distributing information globally, through the entire brain, to cover all its structures within synchronized time patterns. With this, it is already possible to have a truly integrated neural system that regulates a large cognitive domain, within which attention processes, alertness, motor programming, and memory and emotion processes are considered. With this, the individual is capable of having a unique neurophysiological substrate and that allows him to generate different cognitive abilities that in turn allow the appearance of other higher-level behaviors such as language, thinking, and social cognition38.
Then, we have a third organizational loop, constituted by the so-called negative task networks, which dominates the well-known self-referenced thoughts and is linked to the posterior cingulate cortex and the precuneus39. With this, this third loop is constituted as an important brain region in the experience of consciousness5. Other structures participating in the processing of consciousness have also been proposed and are shown in the following figure 1.

Figure 1 Schematic showing the neural correlate of consciousness (NCC). The participation of the paraventricular nucleus (PVT) in the arousal phenomenon is shown, as well as the posterior cerebral cortex, which is related to consciousness understood as content experience. Other participating structures are: the claustrum (CLA) (key zone in the consciousness loop and the transmission of control information), the thalamus (Th), and the third ventricle (V3) (taken from Zhao et al.40)
Finally, a fourth loop of neural networks is considered, which involves tasks of greater cognitive demand and is known as positive task networks41. Normally, these positive task networks, when executing tasks of greater cognitive demand, superimpose their activity states to the negative task network when the subject must process information from outside to generate conscious strategies42. With this, important brain mechanisms such as the central executive network, the dorsal attentional network, and the relevance assignment network are constituted as systems that dominate the previously described43. In this way, another interesting dimension arises, where the prefrontal cortex becomes not only a regulator of emotions or impulses but also as a brain network that has even broader attributes, among which are the formation of concepts and introduction to conscious action plans44. Even the experience of consciousness would also be determined by the levels of connectivity that the prefrontal cortex establishes with the hindbrain45. This is how attention and consciousness are two phenomena that are closely related. Attention alone is not enough to specify the conscious representation of content, but it is a fundamental cognitive resource for conscious access. There is evidence that phenomenal attention and awareness involve synchronized and overlapping patterns of neural activity in the parietal and temporal cortices. It has also been seen that there are representations distributed in many places in the brain, with neuronal activity whose discharge converges mainly in the parietal, temporal, and occipital areas46 (Fig. 2).

Figure 2 Lateral schematic of the brain showing the dorsal attentional network (DAN) (red) and the ventral attentional network (VAN) (green). FEF (front eye field) is also shown; SPL (superior parietal lobule); IFG (inferior frontal gyrus); IPL (inferior parietal lobule); MFG (middle frontal gyrus); STG (superior temporal gyrus); and TPJ (temporoparietal junction) (taken from Nani et al.47)
The neurochemistry of consciousness has been widely discussed and many neurotransmitters have been involved in the process, such as glutamate, acetylcholine, gamma amino butyric acid (GABA), norepinephrine, dopamine, and histamine. Glutamate is seen to be extensively involved in the control of the sleep-wake system. For its part, acetylcholine would participate in memory processes. GABA acts in the cerebral cortex and also in the regulation of the sleep-wake cycle. Norepinephrine is also involved in the sleep-wake cycle, the regulation of moods and attention. Dopamine broadly influences the functioning of the system related to motivation and initiative, along with its impact on the functioning of the prefrontal cortex. Histamine favors the appearance of drowsiness if H3 receptors are activated, while if H1 receptors are activated, alertness will be facilitated48 (Fig. 3).
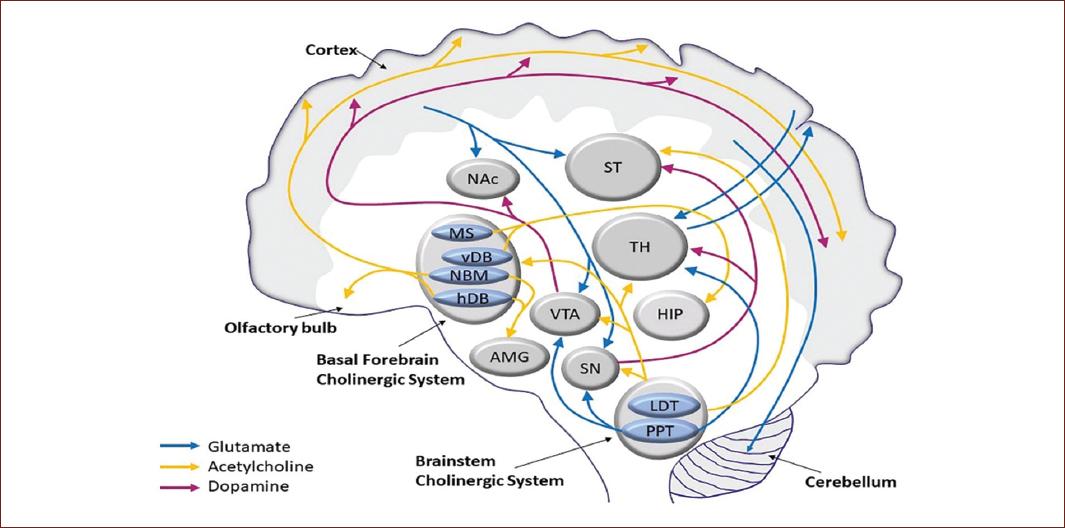
Figure 3 Diagram showing the functioning of the dopaminergic, GABAergic, and cholinergic circuits linked to consciousness. Different structures where neurotransmitters act are also shown. AMG: amygdala; HIP: hippocampus; LDT: laterodorsal tegmental nucleus; MS: medial septum; NAc: nucleus accumbens; NBM: nucleus basalis of Maynert; PPT: pedunculopontine tegmental nucleus; SN: substantia nigra; ST: striatum; TH: thalamus; VDB: vertical limb of the diagonal band of Broca; VTA: ventral tegmental área; and HDB: horizontal limb of the diagonal band of Broca (taken from Gasioroswka et al.49).
Brain phenomena attributed to self-awareness
One of the phenomena most considered lately is not based solely on analyzing the conscious experience or the state of consciousness itself, but also with regard to self-consciousness. This concept refers, in the words of George Prigatano, as “the ability to perceive oneself in relatively objective terms while maintaining a sense of subjectivity”50. In this sense, considering the brain within a context of emergent properties, it has been pointed out that the prefrontal cortex houses important neural networks linked to self-awareness and, specifically, areas such as the anterior cingulate, the orbitofrontal area, and the subcortical regions51.
As indicated above, there are some solid arguments that support the neuronal correlate of self-awareness, among which are noted: 1) that the prefrontal cortex receives projections from all sensory regions that receive conscious experiences and also from somatosensory areas that involve bodily states past and present. 2) which also represents categorizations of the various situations in which the subject is located, classifying the contingency of the life experience51.
With this, it is also possible to appreciate a series of other neuronal structures according to other models that have emerged in this regard. For example, in the anterior cingulum and in the frontoinsular cortex, a fairly specific neuronal type is located, called von Economo neurons or also known as spindle neurons, which are characterized by their fusiform structure and their polar shape. They have not only been found in humans and hominids in general but also in whales and elephants52. The interesting thing about this type of neurons is that they are only found in species that are identified in a mirror and that have certain social structures, such as those previously mentioned. If these neurons were lost, individuals would have difficulties related to the loss of emotional consciousness and some behavioral disturbances in fronto-temporal dementias51.
The role of the default network in consciousness processes
To begin with, it is necessary to point out that this neural network has been extensively documented. It was considered to call default network, or neural network by default, to a series of brain areas that appeared “deactivated” or, what is better, with low states of activity when a subject was directing his behavior toward a specific cognitive task that required high levels of attention. The network described by Raichle53 considered structures as diverse as the ventromedial prefrontal cortex, the dorsolateral prefrontal cortex, the posterior cingulate cortex, the precuneus, and the lateral parietal cortex. In addition, the entorhinal cortex and the hippocampus have been linked to this network54.
Some studies have proposed that this network would have a quite relevant role in processes related to consciousness. For example, it has been shown that the default network could become the neural substrate for the Freudian secondary process55, thereby generating important bases for understanding concepts related to this area of psychology. Another study, conducted in 2014 by Gao and his team, indicated that during the first 6 months of a subject’s life, there is a considerable increase in neuronal connectivity between regions of the hippocampus/parahippocampus, inferior parietal lobe, and the posterior cingulate cortex. This finding accounts for the advances of self-awareness, that is, of becoming aware of oneself, an element linked to primary consciousness and which is closely linked to sensory activity56.
An important mention of the relevance of the default network in the conscious process is with regard to attention-deficit/hyperactivity disorder (ADHD), in which there is a significant imbalance in the cognitive and behavioral control mechanisms57. This is where the default network acquires a special prominence since some of its components, such as the posterior medial or precuneus region, are especially sensitive to distracting stimuli for the subject. The cognitive engagement network also participates, that is, that network that actively participates in the performance of cognitively demanding tasks and in which the dorsolateral prefrontal cortex, frontal ocular fields, supplementary motor areas and the lower parietal lobe participate58. With this, it can be seen that in ADHD the attentional levels, linked to consciousness, being low, would be due to a state of excessive activity of the default network, which would interfere with the consolidation of the cognitive engagement network, generating a low relationship with the environment, and, therefore, suboptimal cognitive performance59.
In recent times, the relationship between the tonic and phasic mechanisms of dopamine release in the brain in conjunction with the work dynamics of the default network and the cognitive engagement network has turned out to be interesting. The phasic release of catecholamines and the high state of activity of the cognitive latching network is linked to goal-oriented behavior. On the other hand, the drop in tonic signaling levels in conjunction with the default network can be related to states of rest and distractibility58, thereby decreasing levels of consciousness. In resting conditions, dopaminergic signaling helps to facilitate the synchronization of the frontoparietal network with the default network, reducing the synchronization of the first network with the cognitive latch network60.
Projections toward the future: relations with artificial intelligence
A wide debate that has been generated in recent times also refers to the relevance that the conscious process has within artificial intelligence.
Machines are known to differ from living beings in many ways, including, for example, the ability to reproduce. Within the diversity of individuals that make up a system is also the singularity and specificity of each of them and, according to that, if we could give our brain a leading role within this specificity, it is possible to show that our brain is in a quite advantageous condition in terms of recognition of patterns, shapes, versatility in relation to computer systems made by man, and except for specific tasks of processing more complex data and which we use to support our daily tasks61.
The question is whether it will definitely be possible to give consciousness to man-made machines. Consciousness, being linked to the concept of intelligence, would reveal the fact that by existing machines equipped with higher levels of consciousness, and secondary to this, intelligence, it could enter a stage of accelerated growth of intelligence, considering as The only limit is what is established in terms of the storage and processing capacity of different data according to what nature dictates62.
There is one aspect that clearly makes the difference: the human being is an individual with characteristics dominated by socialization. Even if it was assumed that a computer could develop self-awareness skills, even if the latter could be modified from different experiences, it would be quite limited by the social aspect. Human socialization would continue to be very different from that of the machine. The other relevant element is the presence of language, an element that in a machine is reduced exclusively to functional terms and linked only to immediate goals, without adding components of the state of knowledge and the expectations of the recipient of the message63.
At present, some models have been defined that try, in one way or another, to solve this problem. One of them is Machine Learning or Automatic Learning, where machines are asked to learn by themselves, to self-program, and to learn from their own experience. This is seen very often in the various systems of the Internet, for example. This has given way to other models such as Deep Learning, where algorithms are capable of learning without human intervention to regulate this process. In this way, they achieve conclusions about the semantic aspects involved in the different data they process. It is here that the elements most similar to the organization of the human nervous system are used, working on the basis of different layers of process units, called artificial neurons, which focus on defining and detecting certain characteristics present in objects64.
Given the dizziness of the development of information technologies, the results regarding these advances keep us expectant. Will machines definitely be entities with levels of consciousness similar or even higher than that of human beings? Can they replace us in our daily tasks or in the world of work? These are questions that will surely be answered as the discoveries are generated.
Conclusion
In this article, we have reviewed the main models that currently govern the processing of consciousness within the strictly neuroscientific and arriving at some approaches related to artificial intelligence. The importance of understanding these phenomena can give way to complement the existing knowledge in neuroscience about human behavior and even link them to the educational field, thereby contributing to improve peoples learning conditions. The issue is complex, much remains to be learned and it is hoped that the various questions raised may be clarified thanks to the scientific advances that may arise in the future in the area.











 nova página do texto(beta)
nova página do texto(beta)


