Introduction
Parkinson’s disease (PD) is a complex neurodegenerative disorder characterized by a wide range of motor and non-motor symptoms.1 Research in PD has mainly focused on the investigation of possible neuroprotection, disease modifiers, and management of motor and non-motor symptoms2-4. Recently, a few number of studies have gone beyond and investigated emotional wellness of the affected people, specifically how persons with PD feel about their own lives5. Well-being can be defined as the experience of health, happiness, and prosperity and is distinguished two components of well-being. The psychological component refers to inter- and intrapersonal characteristics such as autonomy, environmental mastery, personal growth, personal relationships, purpose in life, and self-acceptance that could help individuals to achieve an optimal level of functioning in their daily life6,7. The subjective component reflects the cognitive component of satisfaction with life as a whole, and the presence of positive and absence of negative emotions as affective components8,9. Most studies used life satisfaction as an indicator of well-being. Life satisfaction (LS) is defined as a global assessment of person’s quality of life according to his own criteria10. There is an increasing interest in studying life satisfaction as an indicator of subjective well-being in persons with PD11. Assessing LS could be key in persons with PD as it has significant influence on the process of living with PD and can serve as an indicator of quality of life12,13.
The satisfaction with life scale (SWLS) in persons with PD is a valid instrument for measuring LS in PD. SWLS has shown good convergent validity with other LS scales and good internal consistency and reliability14. The SWLS is considered a psychometrically comprehensive and suitable tool to assess LS in persons with PD15,16. A recent study utilizing the SWLS analyzed factors associated with LS in persons with mild-to-moderate PD, reporting significant correlations of LS with individuals sense of coherence and disease duration17. Other instruments have been developed to measure LS and have been utilized in few research studies of patients with PD. In general, these studies have reported significant associations of motor and non-motor symptoms and of employment status with individuals LS18-21. However, questions regarding the impact of other clinical characteristics which may also affect patient’s quality of life or LS in persons with PD remain unanswered.
Therefore, the aim of this study was to determine the correlation between dopaminergic-induced complications, including dyskinesias and motor fluctuations, with LS in persons with PD. We hypothesize that dopaminergic-induced motor complications will have a negative impact on patients LS. We also investigated the relationship of non-motor and motor aspects of experiences of daily living, and of motor symptoms with their LS.
Materials and methods
An observational cross-sectional study to investigate the association of dopaminergic-induced complications of patients with PD with their LS was carried out. Consecutive subjects were recruited from two movement disorders clinics in Mexico, including the National Institute of Neurology and Neurosurgery in Mexico City and Tecnologico de Monterrey in Monterrey, during the time period comprised from May to October 2019. Subjects included had a clinical diagnosis of PD made by a neurologist with training in movement disorders using the movement disorders society (MDS) clinical diagnostic criteria for PD22. Patients were excluded if they were not able to answer the questionnaires.
Demographic variables, including gender, age, education, marital status, living condition, employment status, geographic region, comorbidities, physical activity, recreational/social activities, and physical therapy were recorded. Clinical variables, including age at symptom onset, age at diagnosis, disease duration, PD motor subtype, Hoehn and Yahr (H&Y) stage, Schwab and England (S&E) scale score, and the MDS-Unified Parkinson’s Disease Rating Scale score (MDS-UPDRS), were also collected. All patients provided a written informed consent and the study was approved by an Institutional Review Board.
Outcome scales
To assess the dependent variable LS, we utilized the Spanish version of the SWLS23. The SWLS evaluates LS as a global summation of an individual’s life situation, inquiring about individual’s subjective evaluation of ideal life, a wish for change, and satisfaction with past and current situation24. It consists of five questions rated on a seven-point Likert scale ranging from “strongly disagree” to “strongly agree.” The responses are added to a total sum score that ranges from 5 to 35 points.
The MDS-UPDRS is a comprehensive scale assessing both motor and non-motor symptoms associated with PD25. It is divided into four parts with a total summed score. Part I evaluates the non-motor experiences of daily living; part II evaluates the motor experiences of daily living; part III consists of the motor examination which was obtained during off medication periods; and part IV evaluates motor complications. All items have five possible responses ranging from 0 = Normal, 1 = Slight, 2 = Mild, 3 = Moderate, and 4 = Severe. Higher scores indicate greater impact of PD symptoms.
To analyze the main independent variable dopaminergic-induced complications, we utilized the MDS-UPDRS scale part IV. To assess the time spent and the functional impact of dyskinesias, we used items 4.1 and 4.2, respectively, and to assess the time spent in the off state, the functional impact of fluctuations, and the complexity of motor fluctuations, terms 4.3, 4.4, and 4.5 were used, respectively. The MDS-UPDRS part I and II total scores were used to determine the non-motor and motor aspects of experiences of daily living of patients with PD, respectively. The MDS-UPDRS part III and the PD motor subtype were used in the study to determine the characteristics of the motor symptoms of patients with PD. The motor subtype was obtained by summing together the MDS-UPDRS items within each subtype, including tremor-dominant (TD), postural instability and gait disorder (PIGD), and indeterminate (IT), to obtain a subscore for each subtype. The final subtype was determined based on ratios of subtype scores26. Then, if the TDsubscore/PIGDsubscore was > 1.15, the TD subtype was assigned, if it was < 0.9, the PIGD subtype was assigned, and if the ratio was in between 0.9 and 1.15, the IT was assigned.
Statistical analysis
Measures of central tendency and variability were used for descriptive statistics. The assumption for normality was evaluated using the Shapiro–Wilk test. A Spearman’s rank-order correlation coefficient was used to assess correlation between independent continuous variables (age, age at onset, age at diagnosis, education, disease duration, S&E, H&Y stage, and MDS-UPDRS scores) with the dependent continuous variable SWLS score. A Mann–Whitney U-test was used to assess the difference between two groups of independent categorical variables (gender; marital status; living status; employment status; chronic diseases; physical activity; social/recreational activities; rehabilitation therapy; and PD motor subtype) and dependent continuous variable (SWLS score). To evaluate the difference between three or more groups of independent categorical variables (geographic region) and dependent continuous variable (SWLS score), a Kruskal–Wallis was used. A partial correlation coefficient was then utilized to estimate the correlation between the independent variables (MDS-UPDRS items 4.1, 4.2, 4.3, 4.4, and 4.5) with the outcome variable (SWLS score) after controlling for possible confounding variables, which were selected if p < 0.05 from univariate analyses. The IBM Statistical Package for the Social Sciences software version 25 was utilized for analysis.
Results
A total of 68 subjects with PD were included in the study. Main demographic and clinical characteristics are presented in tables 1 and 2. The mean SWLS score was 25.7 (SD = 5.3), with most of them reporting a high score 42.6% (n = 29), and a very high score 23.5% (n = 16) regarding satisfaction with their lives. Figure 1 shows a divided bar chart showing proportions of the SWLS scores in our PD cohort.
Table 1 Correlations of demographic variables with satisfaction with life scale scores
| Variables | n = 68 | SWLS, mean (SD) | p value | Effect size |
|---|---|---|---|---|
| Age, years (SD) | 64.9 (13.0) | 25.7 (5.3) | 0.194 | rs = 0.160 |
| Gender | 0.672 | rpb = −0.020 | ||
| Female, n (%) | 29 (42.6) | 25.8 (4.3) | ||
| Male, n (%) | 39 (57.4) | 25.6 (5.9) | ||
| Education, years (SD) | 11.8 (4.7) | 25.7 (5.3) | 0.048* | rs = 0.240 |
| Marital status | 0.787 | rpb = −0.018 | ||
| Single, n (%) | 24 (35.3) | 25.8 (5.0) | ||
| Married or cohabited, n (%) | 44 (64.7) | 25.6 (5.5) | ||
| Living situation | 0.434 | rpb = 0.121 | ||
| Alone, n (%) | 12 (17.6) | 24.3 (6.6) | ||
| With company, n (%) | 56 (82.4) | 26.0 (5.0) | ||
| Employment status | 0.181 | rpb = 0.147 | ||
| Employed, n (%) | 24 (35.3) | 26.8 (5.2) | ||
| Retired/unemployed, n (%) | 44 (64.7) | 25.1 (5.3) | ||
| Comorbidities, n (%) | 32 (47.1) | 25.2 (5.7) | 0.518 | rpb = −0.099 |
| No | 36 (52.9) | 26.2 (4.9) | ||
| Physical activity, n (%) | 37 (54.4) | 26.7 (5.4) | 0.060 | rpb = 0.208 |
| No | 31 (45.6) | 24.5 (4.9) | ||
| Recreational/social activities, n (%) | 16 (23.5) | 27.1 (5.7) | 0.182 | rpb = 0.144 |
| No | 52 (76.5) | 25.3 (5.1) | ||
| Physical therapy, n (%) | 8 (11.8) | 27.4 (5.0) | 0.445 | rpb = 0.116 |
| No | 60 (88.2) | 25.5 (5.3) | ||
| Geographic region | 0.034* | η2 = 0.180 | ||
| Center, n (%) | 38 (55.9) | 24.3 (4.9) | ||
| Northeast, n (%) | 19 (27.9) | 27.2 (5.8) | ||
| Gulf, n (%) | 4 (5.9) | 25.0 (4.5) | ||
| South, n (%) | 3 (4.4) | 26.0 (1.7) | ||
| West (%) | 2 (2.9) | 31.0 (2.8) | ||
| Foreigner, n (%) | 2 (2.9) | 33.5 (2.1) | ||
| PD motor subtype | 0.351 | rpb = 0.154 | ||
| Tremor-dominant subtype, n (%) | 49 (72.1) | 25.2 (5.1) | ||
| PIGD or indeterminate, n (%) | 19 (27.9) | 27.0 (4.4) |
*p < 0.05.
Table 2 Correlations of clinical variables with satisfaction with life scale scores
| Variables | Mean, (SD) | SWLS score | p value | Effect size |
|---|---|---|---|---|
| Age at onset | 56.0 (14.9) | 0.218 | 0.073 | rs = 0.219 |
| Age at diagnosis | 58.9 (13.5) | 0.236 | 0.035* | rs = 0.256 |
| Disease duration | 8.9 (8.4) | −0.203 | 0.059 | rs = -0.230 |
| MDS-UPDRS I score | 9.1 (6.7) | −0.276 | 0.002* | rs = -0.378 |
| MDS-UPDRS II score | 10.9 (7.4) | −0.258 | 0.021* | rs = -0.280 |
| MDS-UPDRS III off meds score | 31.4 (15.0) | −0.149 | 0.072 | rs = -0.220 |
| MDS-UPDRS IV score | 2.6 (3.7) | −0.177 | 0.219 | rs = -0.151 |
| Hoehn and Yahr stage | 2.5 (0.7) | −0.291 | 0.015* | rs = -0.295 |
| Schwab & England score | 73.4 (14.1) | −0.239 | 0.050 | rs = 0.238 |
*p < 0.05. PD: Parkinson’s disease; PIGD: postural instability with gait disturbance; MDS-UPDRS: movement disorders Society–Unified Parkinson’s disease rating scale.
Low-to-moderate statistically significant correlations were observed between age at diagnosis (rs = 0.256, p = 0.035), education (rs = 0.240, p = 0.048), MDS-UPDRS part I score (rs = −0.378, p = 0.002), MDS-UPDRS part II score (rs = −0.280, p = 0.021), and H&Y stage (rs = −0.295, p = 0.015) with SWLS scores.
A Kruskal–Wallis test was conducted to determine the difference between geographic region and SWLS scores revealing a small significant association between variables (h2 = 0.180, p = 0.034). The post hoc analysis revealed that the SWLS scores of patients living in the northeast region of Mexico (27.2, SD = 5.8 vs. 24.3, SD 4.9; p = 0.030) or foreigners outside of Mexico (33.5, SD = 2.1 vs. 24.3, SD = 4.9; p = 0.014) were significantly higher than that of patients living in the center region of Mexico. A Mann–Whitney U-test to determine the difference between other demographic and clinical variables with SWLS scores revealed no significant differences.
A partial correlation coefficient was conducted to estimate the correlation between the MDS-UPDRS items 4.1, 4.2, 4.3, 4.4, and 4.5 scores with SWLS scores after controlling for age at diagnosis, education, MDS-UPDRS part I and II scores, H&Y stage, and geographic region. The analysis revealed a moderate statistically significant correlation between the functional impact of motor fluctuations (MDS-UPDRS part 4.4) and SWLS scores (rs = −0.408, p = 0.001). No significant correlations were observed between the time spent in off state, the complexity of motor fluctuations, the time spent with dyskinesias, or the functional impact of dyskinesias with SWLS scores. Scatterplots depicting these correlations are shown in figure 2.
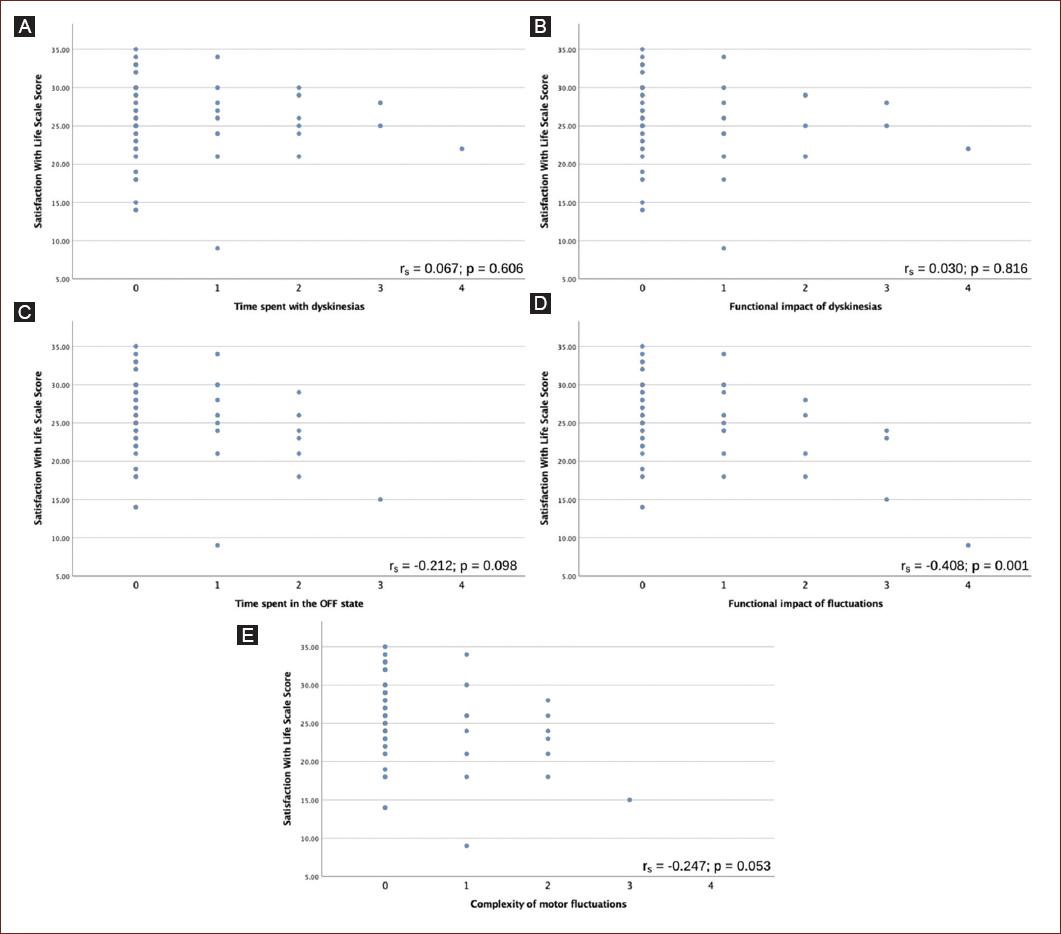
Figure 2 Scatterplot showing correlation between movement disorders society – unified Parkinson’s disease rating scale (MDS-UPDRS) items 4.1-4.4 with satisfaction with life scale 25 (SWLS) scores. A: shows a non-significant zero correlation between time spent with dyskinesias and SWLS. B: shows a non-significant zero correlation between functional impact of dyskinesias and SWLS. C: shows a non-significant negative correlation between time spent in the off state and SWLS. D: shows a medium significant negative correlation between functional impact of motor fluctuations and SWLS. E: shows a non-significant negative correlation between complexity of motor fluctuations and SWLS.
Discussion
We conducted an observational analytical cross-sectional study in a Mexican sample of persons with PD to correlate dopaminergic drug-induced complications and their LS. Our study revealed a statistically significant negative correlation between the functional impact of motor fluctuations (MDS-UPDRS part 4.4) and SWLS scores, when controlling for possible confounding variables. No significant correlations were observed with other characteristics of motor fluctuations (MDS-UPDRS part 4.3 and 4.5) or with dyskinesias (MDS-UPDRS part 4.1 and 4.2).
Our results suggest that motor fluctuations but not dyskinesias, impact on life satisfaction in persons with PD. To the best of our knowledge, this is the first study investigating an association between dopaminergic drug-induced complications and life satisfaction, so comparison with other studies is problematic. Our results are in line with the previous studies which measured quality of life as clinical outcome, reporting that motor complications may decrease patient’s quality of life27. Strategies to reduce motor fluctuations and better understanding the contribution of these to persons’ lives may help minimize the negative impact of these complications on happiness or well-being. In regard to dyskinesias, data have shown that the majority of subjects had a mild and non-troublesome dyskinesias that can be adequately treatable28. Our results suggest dyskinesias may not have as much impact on person’s life satisfaction as compared with motor fluctuations. The lack of significant correlation between dyskinesia scores and SWLS scores may also be explained due to the poor awareness of these involuntary movements patients usually have29.
In addition, we observed higher than average SWLS scores, which are around 26. This is higher than previously reported scores which ranged between 21.1 and 24.214-17. It should also be noted that it has been reported that Hispanics show greater life satisfaction than non-Hispanics possible related to a higher spirituality among Hispanics30.
We also observed significant correlations between ages at diagnosis, years of education, geographic region, and clinical markers of disease severity such as MDS-UPDRS parts I and II and H&Y stage, with SLWS scores in our cohort of Mexican PD patients. Although the majority of the previous studies investigating life satisfaction in patients with PD utilized other scales to measure life satisfaction, our results are consistent with these previous reports stating that patient’s life satisfaction is significantly correlated with non-motor and motor aspects of daily living, with the stage of the disease, and with years with PD diagnosis17-21. Based on these data, providing better control of non-motor and motor symptoms of patients with PD may have a beneficial effect on patients’ life satisfaction or well-being.
Our study is the first to report a significant association between years of education and geographic region with life satisfaction. A previous study has shown that well-being of individuals differed in terms of geographic location, which may be associated to different economic, social, and health indicators31. These information may help clinicians detect those patients prone to not feel satisfied with their own lives. Thus, we should consider these factors when managing patients with PD to enhance their life satisfaction.
Several limitations of the present study need to be mentioned before interpretation of results. The issues related to observational cross-sectional studies such as selection and memory bias need to be considered. The participants might not be representative of the target population. Some data might be inaccurate since the scales utilized to measure outcomes might not have the highest precision. We attempted to prevent possible interviewer bias using different interviewers. Moreover, other factors not analyzed in the study, including missing caregiver’s point of view, might have a confounding effect. Although some of the biases mentioned cannot be controlled, the statistical methods were utilized to control for possible confounders during data analysis.
Conclusions
LS was correlated to the functional impact of motor fluctuations but not to dyskinesias in our PD cohort. Moreover, geographic region, education, and clinical factors related to PD were also correlated to LS. Strategies to reduce the functional impact motor fluctuations and better understanding the contribution of these to patients’ lives may help minimize the negative impact of these complications on patient with PD.











 nova página do texto(beta)
nova página do texto(beta)



