Introduction
Neurodegenerative diseases (NDDs) are characterized by a progressive dysfunction and loss of vulnerable neurons and are the leading cause of disability and morbidity worldwide1. Neuronal cell death is the final outcome when multiple chronic and progressive stressors pile up beyond the neuron´s recovery capacity, resulting in neurodegeneration2. However, a traumatic incident or acute event like stroke can cause a sudden decline of energy and a pro-inflammatory environment in the affected neurons, which also results in acute cell death and brain tissue degeneration2. Thus, we can conclude that NDDs are not strictly chronic neuronal death pathologies, but also involve acute processes.
The gutbrain axis (GBA) refers to the set of biological systems which creates a network connection that allows bidirectional communication between gut microbiota and diverse areas on the brain and the SNC3. GBA interconnection routes such as the vagus nerve, the immune system, the hypothalamic-pituitary-adrenal axis (HPA), tryptophan metabolism, and the enteric nervous system4. When there is an aberrant response from the normal microbiota or it has suffered a number of alterations, an inflammatory response takes place, resulting in the liberation of cytokines and microbial metabolites which can lead to a certain level of neuroinflammation5.
Gut microbiome plays a crucial role in neurodevelopment, as well as in several brain diseases6. Intestinal microorganisms have an impact on the host´s metabolism and immune status, which affects neuronal pathways in the enteric and central nervous systems3. Moreover, it has been recently demonstrated that poor communication between gut-brain axis networks as well as gut microbiota disturbance can lead to gut inflammation disorders, and consequently alter cognitive functions, favoring the development of acute and chronic neurodegenerative conditions7. In this text, we aim to describe the influence of GBA in three specific neurodegenerative pathologies (Stroke, Alzheimer’s disease [AD], and Parkinson’s disease [PD]), as well as highlight the importance of healthy GBA status as possible preventive and active treatment for NDDs.
Alzheimer
AD represents a multifactorial slowly and progressive neurodegenerative disorder characterized by a progressive cognitive decline as well as by a gradual impairment in emotion, language, and memory8. It represents the leading cause of dementia worldwide9. According to Mexico’s National Institute of Neurology and Neurosurgery, AD’s prevalence is around 350,000 people, of which 2,030 die each year. The lack of official data by the Mexican health system makes it difficult to elucidate other statistics related to this disease, however, a work carried out by Gutierrez-Robledo and Arrieta-Cruz in 201510, shows that AD has an incidence of 27.3 (1000 people/year) and a greater predisposition for women over 60 years of age.
The pathogenic hallmarks of AD include extracellular aggregates of amyloid β (Aβ) plaques and intracellular accumulation of neurofibrillary tangles (NFTs), composed of the hyperphosphorylated tau protein11. Abnormal cleavage of the Aβ precursor protein, a membrane integral protein, by secretase enzymes5, results in the formation of insoluble Aβ fibrils, which are then excreted into the interstitial fluid and start to oligomerize and aggregate into plaques, diffusing into synaptic clefts and thus interfering with normal synaptic signaling11,12. Aβ aggregates begin to form in the neocortex and appear later in the hippocampus4. The tau protein is widely distributed throughout neurons in association with microtubules, playing a critical role in cytoskeletal integrity and axonal transport13. Polymerization of Aβ fibrils, in turn, leads to abnormal activation of several kinases, causing hyperphosphorylation of tau and a subsequent polymerization into insoluble NFTs211. The typical clinical course of AD can be divided into 4 stages. AD symptoms usually develop once both Aβ and tau aggregates are found in the neocortex12,14,15.
AD remains being one of the diseases with the lowest number of pharmacological therapeutic options. As of date, the first-line treatment option for AD remains to be cholinesterase inhibitors, including donepezil, galantamine, and rivastigmine15,16. As of June 2021, a new drug option received FDA approval for AD treatment: Aducanumab (Aduhelm)17,18, a human gamma immunoglobulin 1 monoclonal antibody targeted against Aβ, which should be initiated in the early stages of the disease, when the patient’s cognitive impairment is still mild17, however, it’s long-term tolerability and security is currently under evaluation in a multinational phase 3b clinical study.
Parkinson
PD; one of the most common and intricate neurological disorders in clinical medicine, is a progressive NDD caused by a dopamine deficiency throughout basal ganglia, secondary to the premature death of dopaminergic neurons in the substantia nigra pars compacta. Resulting in a movement disorder with marked parkinsonian motor symptoms18. Although there is a gap in the epidemiological information of PD in Mexico, there have been estimations for a prevalence of 40-50 cases/100,000 inhabitants a year, additionally, it’s reported to be the fourth cause of consultation in the National Institute of Neurology and Neurosurgery19.
Globally, PD represents the second most common neurodegenerative disorder20, caused by multifactorial etiology that combines environmental and genetic factors, however, its presentation is linked to specific disease hallmarks, firstly, a degeneration of dopaminergic neurons in the substantia nigra, recent studies have shown that loss of the dopaminergic terminals in the striatum directly correlates with motor symptoms onset. And secondly, sporadic PD pathognomonic intraneuronal aggregates known as Lewy bodies and neurites21: eosinophilic cytoplasmic inclusion bodies with abundant alpha synuclein20, located in first stage pathology medulla oblongata that consequently spread through neural pathways to the substantia nigra in latter phases. There are genetic factors related to the pathogenesis of PD, with roles in mitophagy, protein degradation, increase kinase activity, the key genes being: PARKIN, PINK1, DJ-1, LRRK2, and GBA21. As a result of such pathogenesis, PD presents motor and nonmotor symptoms. Classical motor findings list resting tremor (often unilateral), postural instability, rigidity, and bradykinesia, furthermore, speech turns softer, swallowing gets affected, sialorrhea and difficulty rising from a chair might present due to the slowing of movement, whereas nonmotor symptoms include cognitive decline, sleep disturbances, dysautonomia, anosmia and even psychological and gastrointestinal alterations such as anxiety, depression, nausea, bloating and abdominal discomfort22.
Currently, there is no curative treatment for PD, thus, it focuses on decreasing the degree of any symptoms developed by the patient. The best treatment plan includes levodopa administration, the immediate precursor of dopamine20.
Stroke
Stroke accounts for the second leading cause of death21. It is defined as a neurological event in which abrupt impairment of blood perfusion through cerebral vessels to the brain accounts for a neurological outburst22. In Mexico, stroke does not only represent an important source of disability and mortality for its population but a great threat to family conformation and dynamics23. Epidemiological information suggests a rise in the prevalence of stroke, in 2008 stroke scaled up from the fourth cause of general mortality (2000) to the third with more than 30,000 deaths in that year24. After analyzing verified stroke cases in 2011, estimations were made, it was concluded that the prevalence was 5.1 every 1000 inhabitants while the accumulated incidence showed 232.3/100,00023.
Stroke can be divided into ischemic and hemorrhagic, with the ischemic variant accounting for the vast majority of cases25. The ensuing insufficient cerebral blood flow and the subsequent lack of oxygen supply to the brain leads to cell death by various mechanisms such as ionic imbalances (calcium overload), glutamate excitotoxicity, bloodbrain barrier breakdown, activation of microglia with the subsequent neuroinflammation, cytotoxic and vasogenic edema, dysregulation of energy metabolism and oxidative stress21, leading ultimately to necrosis of the cerebral parenchyma. It has been recognized that Stroke harmful effects consist of two principal injuries First injury alludes to the direct effects of ischemia and necrosis to brain tissue, second injury refers to the deleterious effects of persisting neuroinflammation several days after the stroke took place, this persisting inflammatory response might be active even months apart from the onset event26. Necrosis and apoptosis-mediated cell death drive an inflammatory reaction, in which innate and adaptive immune cells release a large amount of proinflammatory cytokines, such as tumor necrosis factor-alpha, interleukin (IL) 1 beta, IL-6, and interferon-gamma, which exacerbate the initial damage, manifested by a larger ischemic area and a worsening of symptoms in patients27. In addition, there is an activation of microglia and astrocytes by the presence of inflammatory mediators28.
Clinical manifestations in stroke patients vary in presentation, severity, and structure due to variables such as onset, affected area, and etiology, thus, it’s identification and diagnosis can be misleading and complicated for physicians26, nevertheless, an acute onset of signs like hemiparesis, aphasia26,29, gait disturbances, eye movement abnormalities, visual field abnormalities and symptoms such as subjective weakness, paresthesia, headache and dizziness with additional focal neurologic deficit may assist examiners in the early recognition of a patient developing a stroke29.
GBA and NDDs
Microbiota is a symbiotic population of bacteria, viruses, fungi, and other microorganisms that coexist in the gastrointestinal tract30. The question remains “how can microbes in the gut have an effect in the brain?” There are many potential linking pathways, such as the autonomic nervous system connecting through the 10th cranial nerve, the vagus, (specifically the hepatic and celiac branches) the gut, and the brain with neuronal and neuroendocrine signals bidirectionally31. Other pathways involve the HPA, altering the microbiota in stressful situations by releasing molecules such as norepinephrine, catecholamines, serotonin, and cytokines in the enterochromaffin cells in the gut, short-chain fatty-acids transported to the brain in the vagus nerve and/or the systemic circulation, stimulating the neurons, microglia, astrocytes, and bloodbrain barrier32.
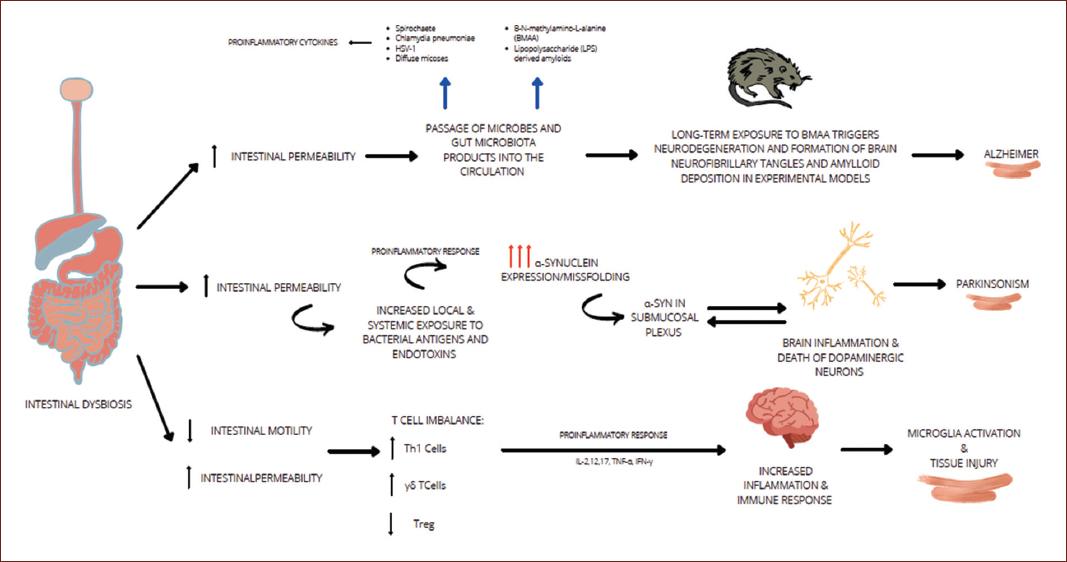
As summarized in Figure 1, neurological disorders, especially those presenting with neurodegeneration, have been associated with harmful environmental factors identified in childhood; in particular, an unbalanced diet that alters early gene expression leads to epigenetic changes that manifest in adulthood. Research suggests that Gut dysbiosis increases gut permeability causing a bacterial leak that triggers an inflammatory response. Proinflammatory cytokines are released, which increases the blood-brain barrier permeability, inducing neuroinflammation32. It’s known that common bacteria present in the gut microbiota such as Lactobacillus, Bifidobacteria,nterococcus, and Streptococcus species produce neurotransmitters like acetylcholine, GABA, and serotonin in a high scale, for example, 90% of the serotonin for brain and gastrointestinal tract use is produce in the gut31,32. This is important because serotonin binding to 5-HT receptors on microglia induces the release of cytokine-carrying exomes, therefore modulating a gut-induced neuroinflammation5.
GBA and AD
The mechanisms of the brain-gut-microbiota axis related to the pathogenesis of stress conditions or brain disorders are being discovered. Inflammation and Alzheimer disease have been studied for their relation, due to the increased amyloid deposition under inflammatory conditions. Microbiome studies point out that a variety of microbial species, including Enterobacteriaceae and Lactobacillaceae families generate significant quantities of functional amyloid33. These microbial species increase on the gut as the human gets older, and with them, the quantity of functional amyloid34. Amyloid neurotoxic properties make older people more susceptible to gut dysbiosis, and because of the GBA it is suggested that it can increase the risk of developing AD33,34.
Over the last years, several studies have identified that the group of bacteria mentioned above, may also produce damage to gut epithelium, which increases its permeability and the leak of bacteria to the blood system34. Gut microbiota or toxic metabolites can then travel to the brain and, under other proinflammatory conditions or immune system depression (like the physiological immune system capabilities reduction that occurs in the elder) penetrate the bloodbrain barrier and induce neuroinflammation. Nevertheless, more research is needed in order to identify specific bacteria or metabolites that might be involved in AD pathophysiology35.
GBA and PD
The pathogenic mechanisms underlying neurodegenerative disorders such as PD is attributable to multifactorial changes. Emerging data has confirmed that imbalances on the GBA trigger or exacerbate the progression of PD36. An important hallmark on PD is the a-synuclein (aSyn). aSyn is a protein consisting of 140 amino acids that play an important role on synaptic plasticity and interact with presynaptic vesicles. Several research studies have studied the aSyn aggregates in the GI tract. Phosphorylated aSyn deposition has been observed on subjects with idiopathic rapid eye movement sleep behavior disorder, a prodromal marker of PD, as well in gastric, duodenal, and colonic biopsies undertaken by PD patients before the presentation of motor signs37,38.
The innate immune system, the first line of defense against invading microbes, can sense the presence of microorganisms through a number of pattern-recognition receptors (PPRs), which recognize pathogen-associated molecular patterns. Among the different PPRs, Toll-like receptors (TLRs) stand out on PD and GBA relations. TLR-4 has appeared in several studies interacting with aSyn, triggering microglial responses38,39.
An existing hypothesis suggests that the vagus nerve is a potential pathway of retrograde transport of aSyn between the enteric nervous system and the brain. Enteric dysregulation can contribute to PD pathogenesis due to the increase on intestinal epithelial barrier permeability40. The association between inflammatory bowel disease and PD can be another role of the intestinal inflammation. Alteration on PD patient’s microbiomes has shown decreased abundance of genus Prevotella, Roseburia and Blautia genera, Lachnospiraceae family, among others, and an important increase on Lactobacillaceae family41. With this new perspective focusing on the microbiome, PD can have a new understanding, providing new therapeutic approaches involving the GBA.
GBA and ischemic stroke
Existing evidence has shown how ischemic stroke is capable of causing an alteration on the gut microbiota. After stroke, an inflammatory response takes place principally induced by DAMPs and cytokines release28. BBB suffers damage and a bidirectional communication with gut microbiota enhances the immune response. The changes in BBB allow inflammatory and immune cells from the circulation to get into the brain parenchyma, which interacts with innate immune cells in the CNS. Furthermore, gut inflammation (initially induced by the stroke) can confer systemic inflammation that contributes to brain inflammation28,29.
Direct neural inflammation produced by the ischemic event, with the addition of systemic inflammation due to microbiota dysbiosis potentially enhances the harmful effects of stroke and increases final neurodegeneration of the secondary injury. Similarly, recent research indicates that pro-inflammatory gut microbiomes may be related to worst prognosis after stroke likely due to a heightened immune system that generates detrimental pro-inflammatory response after cerebral ischemia42.
Novel therapies targeting microbiota induced neuroinflammation are being developed43. The inhibition of intestinal IL-17 secreting γδ T-cells by Treg cells may reduce poststroke inflammation in mice44. Th17 cells are used at a significant part in keeping mucosal barrier, inflammation, and microbial translocation in the gut and have the capacity to efficiently break the BBB to penetrate in the CNS. Inhibition of Th17 cytokines may be chosen to reduce inflammation44. The novel transgenic models can help for the identification of the origin, the mission, and fate of gut migrating immune cells in stroke.
Conclusion
NDDs have a strong relationship with gut microbiota in their pathophysiology. Inflammation appears to be a constant link between the gut and brain that encourages the development of these diseases. GBA is a field of study that must be deeply analyzed in order to better understand NDDs and to be able to propose new therapies that help not only to treat but also prevent NDDs.











 nueva página del texto (beta)
nueva página del texto (beta)