Introduction
We propose the novel term “Somatoapraxia” to be the alteration in somatosensory and proprioceptive processing, after acquired brain injury, which makes it problematic to manage the body schema and carry out the necessary postural adjustments for the execution of tasks or actions. It differs from apraxia, since apraxia has been defined as a disorder that involves the inability to execute learned and purposeful movements, on command or by imitation, due to alteration in the concept of movement or in the logical motor sequence of previously learned movements1,2.
In adults, different descriptive models for apraxia have been proposed, which encompass different etiologies: failure in visual input, memory, semantics, or in the management of space and time3. In children with learning disabilities, Ayres proposed a similar term, somatodyspraxia4, to refer to the inability to interpret and organize sensory information that allows an adequate and effective response to multiple environmental stimuli5. However, this only considers the origin of the problem to be due to planning and conceptualization factors of ideational apraxia. Furthermore, in adults with acquired brain injury, alterations in sensory and proprioceptive processing that impact daily living activities have been described1; however, they have not been considered as part of a theoretical model.
A somatosensory and proprioceptive core concept, which explains the complexity of the different types of apraxia after acquired brain injury, has not been proposed. Therefore, is our objective to present the term “Somatoapraxia” as a novel core concept that exists as principal factor in apraxia. We will also highlight the terms applicability in a Neuropsychological Rehabilitation Model for apraxia.
History of apraxia
The term apraxia was proposed by Steinthal in 1871, to refer exclusively to the set of disorders that had as the common characteristic, the inability to correctly execute a motor activity on command6. However, the most important person responsible for its recognition was Hugo Karl Liepmann (1863-1925), who distinguished different functional components responsible for praxis processing. He believed that the learning of motor skills required the acquisition of “movement formulas,” “innervation patterns,” and “kinetic memories”3. Thus, Liepmann characterized ideational apraxia, ideomotor apraxia, and limb or kinetic apraxia as consequences of brain injury in adults7. Liepmann proposed ideational or conceptual apraxia as an interruption in the activation of the space-time plan, therefore, the idea of movement is not possible. Conversely, in an ideomotor apraxia the spatio-temporal plans are preserved, but they cannot guide the innervation engrams to execute movements because they are disconnected from them; the patient knows what to do, but not how to do it. Finally, when the interruption of the innervation engrams interferes with the selection of muscular synergies to perform movements, there is kinetic apraxia of the limbs7.
Even then, Liepman considered that praxis requires the idea or plan of movement, which must be recovered and associated through the left sensorimotor cortical connections, which in turn carry information to the left primary motor areas. When the left limb performs the movement, the information is transmitted from left to right through the corpus callosum and activates the right motor cortex8.
Explanatory models of apraxia
In recent decades, complex cognitive models of praxis processing have been developed to explain and classify apraxia. The description of the mechanisms underlying this disorder has been diverse. Heilman, González Rothi and Valenstein9 in 1982, and González Rothi, Heilman, and Warson10 in 1985, proposed that there are at least two types of mechanisms that describe apraxia: (1) a degraded memory trace that produces difficulties in the reception and production of gestures, and (2) a memory discharge dysfunction that generates alterations in their production3.
Roy and Square, in 1985, proposed that praxis processing is mediated by a system that involves two components, both conceptual and of production9. The first includes three types of knowledge: (1) about the operation of tools and objects, (2) about the independent actions of objects, and (3) about the sequential organization of actions. They considered that movements are dependent on the interaction between the conceptual knowledge of the tools, objects and actions (semantic action), and the structural information contained in the motor programs. In this context, apraxia is the result of a failure in the production system, while an alteration in the conceptual system implies difficulties in the recognition of the specific utility of tools and the mechanical requirements of an action that allows us to achieve an objective10,11.
Similarly, a model of apraxia was proposed by Geschwind12 in 1965, based on the disconnection of the left premotor cortex and Wernicke’s area13. According to this model, apraxia is the result of lesions in the supramarginal gyrus or the arcuate fasciculus.
Ayres4 based on her studies in children, identified a population with learning disabilities who showed difficulties in interpreting the sensory information of their bodies and their environment. Based on her research and clinical experience, she described how the nervous system translates somatosensory information into action, and postulates that proper integration of proprioception and the vestibular aspect are critical for adaptive behavior14. Ayres emphasized somatosensation and its relationship with the body schema as the basis for praxis in children; however, she proposes that praxis is based of only three components: ideation (conceptualization of actions), motor planning, and motor execution. Furthermore, she describes somatodyspraxia as a problem in the organization of the motor plan, such as, ideational apraxia.
Although different models have been described to explain apraxia, somatoapraxia has not been considered as a primary factor of the different apraxia. We consider that when somatosensory and proprioceptive processing is altered, after acquired brain injury, the management of the body schema and the necessary postural adjustments to carry out tasks or actions, (praxis), becomes difficult. Based on this theory, we present the term somatodyspraxia as a primary factor in apraxia and its applicability in a Neuropsychological Rehabilitation Model for apraxia.
Somatodyspraxia as a primary factor in apraxia
In somatodyspraxia the ability to execute an action is altered. This ability requires that the brain is capable of knowing bodily functions and conceptualize the objective of a motor action. In somatodyspraxia, the management of the body schema and postural adjustment is absent. In the execution of praxis, the processing of information from the proprioceptive, somatosensory, kinesthetic, and vestibular systems is required to determine how the body is designed and how it will function for the performance of a task or action. This sensory processing allows using exteroceptive and interoceptive information to regulate and adapt movement and give appropriate motor and postural responses15 necessary in performing praxis.
In Somatodyspraxia, the integration of proprioceptive, somatosensory signals, as well as body scheme that allow estimating body movements is altered, resulting in gait apraxia16. The proprioceptive system allows us to perceive the movements of the joints, to know the position of the body, the force generated by the muscles, and the speed and direction of our movements17. System alterations complicate the reception of normal impulses from the muscles and joints18. Processing alterations of proprioceptive sensations result in disruption of postural stability and lack of knowledge of the body’s position in space19,20. In addition, neuronal principles show that for adequate postural control of the head, the interaction of the vestibulospinal tracts, which selectively activate the muscles of the neck and trunk, are crucial21. Information that travels from the skin to the central nervous system (CNS) is useful to generate human balance. When the cutaneous afferent inputs are not transmitted to the CNS, it can cause an imbalance22, and subsequently gait apraxia.
In somatodyspraxia, there is alteration of tactile information, which contributes to the manipulation and grasping of objects, plays a role in linking present and past sensations necessary for the performance of praxis23. These difficulties can lead to apraxic agraphia, in which the subject struggles in the simple task of properly grasping a pencil and guiding movements to trace letters. The same can happen in adult patients with acquired brain injury, who experience difficulties to adequately perform activities of daily living24.
Somatodyspraxia can cause problems in the body schema and in postural adjustments necessary for dressing. The patient may have problems when trying to put on a sweater, having difficulty in placing arms in the corresponding sleeves, for example, because he does not make the necessary postural adjustments. It is also common for the patient to carry out the steps to put on a garment incorrectly or in the wrong order, for example, putting the pants on the arms, or putting on a sweater first and then the shirt, or not knowing whether to tie their shoelaces or button a shirt. In addition, they have difficulty in fastening and unbuttoning25, causing dressing apraxia.
In somatodyspraxia, the internal and updated representation of the position of the body and movement in space are affected26. Clinical observations show that patients with somatosensory and vestibular alterations often make errors in movement trajectory and may have trouble detecting and estimating body displacement26. Signals from muscles, joints, skin, and eyes are continuously integrated with vestibular input and evoke postural and oculomotor responses. Alterations present at this level, can lead to a constructional apraxia, a concept that describes an alteration present when carrying out activities such as assembling, building, or drawing27.
Somatodyspraxia may be the primary factor in limb apraxia, since a patient with acquired brain injury may have problems in the body schema management and postural adjustments to orient utensils and move it properly, or he cannot use the appropriate cutlery for the type of food or associate the object with the action (e.g., using a knife instead of a spoon for soup or adding salt instead of sugar to the coffee). Furthermore, the subject may have difficulties in using objects, such as the toothbrush and razor, trying to use the toothbrush to comb his hair. Furthermore, they may have difficulties in properly orienting and positioning their body, for example, to properly manipulate a hairband to make a ponytail.
Orofacial apraxia, or buccofacial apraxia is characterized by a loss of voluntary control of facial, lingual, and masticatory muscles in association to somatosensory and proprioceptive disturbance. The typical patient fails to produce the correct movement in response to verbal command or to imitate correctly a movement performed by the examiner due to Somatodyspraxia. Sometimes, the person needs to use his hands to help introduce food completely into the mouth and thus be able to swallow. It may also happen that the patient does not find the proper position of the phonation apparatus for the correct articulation of language, producing an afferent motor aphasia27.
Similarly, patients with Somatodyspraxia may experience difficulties in the body schema and sense of the body itself. Compound sensory, proprioceptive, exteroceptive, and interoceptive data, are required for sphincter control28. Like all body Functions, sphincter control is regulated by the Nervous System. At the level of the entire lower urinary tract (bladder and urethra) there are a series of sensory endings or exteroceptive (tactile, painful, and thermal) and interoceptive (visceral or abdominal distension) neuroreceptors located mainly in the trigone and urethral meatus29. Patients barely know what position their body is in and, therefore, cannot receive afferent information from the sphincters.
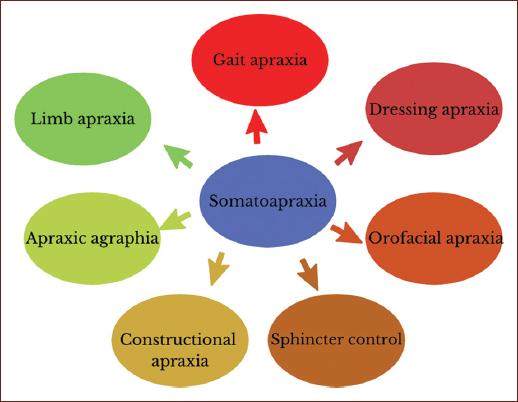
In summary, the functional implications of somatodyspraxia are reflected in different activities such as walking, dressing, the construction of letters and drawings, in the adequate movement of the organs of the mouth for the articulation of language and swallowing, and sphincter control, due to the lack of adequate integration of somatosensory and proprioceptive information, body schema and postural adjustments for the execution of praxis (Fig. 1). The novel term plays an important role in the mechanisms responsible for the previously mentioned apraxias.
Somatodyspraxia in acquired brain injury
In praxis, movements are complex and seem to involve several brain regions, such as the premotor, prefrontal, temporal, and parietal areas. The parietal lobe has been shown to be involved in the acquisition of movements for the use of tools13. In addition, the parietal cortex seems to be relevant in abnormalities of the somatosensory and proprioceptive systems, which have been found altered in disturbances that affect the CNS, such as a cerebrovascular event (stroke)30, Traumatic brain injury (TBI)31, or neurocognitive impairment32. Therefore, the somatosensory and proprioceptive alterations in acquired brain injury lead to the presence of somatodyspraxia.
Because somatosensory and proprioceptive deficiencies are common in somatodyspraxia patients after stroke, deterioration in postural function and level of independence may be present32. They often require assistance during bathing, shaving, and getting dressed33. Neuroimaging studies, which include positron emission tomography and functional magnetic resonance imaging, have shown that lesions to the lower left and upper parietal lobe seem to be involved in the alteration of movements for the use of tools13.
Alzheimer’s disease is associated with somatosensory processing and discrimination32 that can lead to somatodyspraxia which impacts daily living activities, such as meals, housework, or driving. Commonly, in mild cognitive impairment, significant decline in the performance of these activities is observed, which may progress rapidly into Alzheimer’s disease34. The problems in carrying out these activities, observed in this population, may be related to the deterioration in praxis due to a primary somatodyspraxia factor, which causes struggles in action executing such as the use of cutlery or judging how close the food is to the mouth35.
Similarly, patients with Parkinson’s disease experience somatosensory alterations and problems in proprioceptive processing causing somatodyspraxia, due to an affection of the basal nuclei and their connections with postcentral and premotor areas13. These alterations cause lack of precision in goal-directed movements and altered postural reflexes that lead to problems in gait and balance36. We observe temporal or spatial errors, such as interrupted slow movements apraxic substitution errors, such as brushing teeth with a hairbrush, or using a part of the body as an object37.
It is common that after a TBI, patients present disorders of the somatosensory and proprioceptive systems31. Deficiencies in the ability to integrate information from the sensory, motor and skeletal-muscular sywstems can generate somatodyspraxia in patients with TBI38. This causes difficulties in grasping or thumb orientation for proper use of tools, due to alterations in the postcentral and premotor areas of the contralateral hemisphere to the injury39. Therefore, daily living activities become arduous.
Cerebellar ataxia arises due damage or dysfunction affecting the cerebellum and/or its input/output pathways cerebellar dysfunction may result in significant functional difficulties with upper and lower limb movement, oculo-motor control, balance and walking. Contrary to somatodyspraxia, ataxia is associated with dysmetria, cerebellar tremor, nystagmus, and dysarthria. Its rehabilitation is centered in coordination and balance training, multifaceted inpatient rehabilitation, a cycling regime, balance training, treadmill training, occupational therapy, and inspiratory muscle training35.
After brain injury, patients require neuropsychological rehabilitation, which is a theoretical framework of interventions designed to improve cognitive, emotional, and psychosocial functioning, due to changes in the brain. The goal is to promote greater functional independence in a wide variety of situations of daily life40. So far, few studies have investigated the effectiveness of different apraxia methods of intervention. This may be caused by the erroneous belief that apraxia only occurs during imitation, verbal command, and in the absence of the object, without influencing daily life activities41. There are some interventions proposed in the literature41,42, which focus mainly on syndromic management strategies such as ideomotor apraxia, orofacial apraxia, or dressing apraxia. In addition, other rehabilitation techniques have focused on the execution of gestures43. However, improvement was observed only in the symptoms, without generalization. Conversely, Durand, Gago Galvagno and Elgier44, treated neurocognitive ability and not the symptom, they based the rehabilitation on the body schema in patients with dressing apraxia, during which they provide Kinesthetic-tactile, propioceptive and spatial stimuli to guide the upper limb during movement44.
Functional neuroanatomical bases for our somatodyspraxia rehabilitation model
In praxis, movement depends on simple processes integrated and organized in learned motor patterns1. Therefore, execution of a praxis pattern requires a constant flow of information between the cortical and subcortical areas; thereby being a mechanism in which the cortex serves as a station were information is classified and integrated, while the subcortical structures provide feedback to the entire system, rectifying input information and the commands that will result in the execution of specific responses.
The spinal cord acts as a communication bridge between the brain and the sensations received by the body from the external world and the internal environment. Thus, the sensory information of different submodalities is sent to the brain through the spinal tract. Sensory nerve fibers of different sizes and functions are arranged and distributed in nerve bundles or tracts. Below, we show the most relevant afferent pathways that reach the medulla and their function; this, to show the relationships they maintain with the production of action (praxis) and our proposal for neuropsychological rehabilitation of praxis (Table 1). Somatodyspraxia patients show alterations in recognition and association of multiple somatosensory and proprioceptive stimuli. For rehabilitation, we suggest applying tactile stimuli to the subject, such as pain, temperature, pressure, texture and vibration. We also seek to place him in uncomfortable positions and carry out tasks such as pulling, loading and pushing, in order to stimulate the sensations of the muscles and joints leading to the goal of movement and to promote knowledge of the parts of the body to solve a task (praxia)48.
Table 1 Functions and sensations of the Ascending pathways of the spinal cord and proposal for neuropsychological rehabilitation in somatodyspraxia
| Ascending pathways | Sensation | Fiber connections | Terminate in | Proposal for neuropsychological rehabilitation of somatodyspraxia |
|---|---|---|---|---|
| Lateral spinothalamic tract | Pain and temperature | Reticular formation Ventral posterolateral nucleus of the thalamus Somesthetic area (postcentral gyrus of the cerebral cortex) |
Postcentral gyrus | Apply uncomfortable/painful stimuli to feet and hands,
due to their cortical representation Apply ice to the parts of the body where you want to increase muscle tone |
| Anterior spinothalamic tract | Slight touch and pressure | Superior cerebellar peduncle Cerebellum |
Postcentral gyrus | Apply large heavy cushions to the entire body
Use soft objects of different weights as stimuli |
| Gracile and cuneate fasciculi medial lemniscus | Discriminatory touch, vibratory sensitivity, conscious sensation of muscles and joints | Inferior cerebellar peduncle Cerebellum |
Postcentral gyrus | Use objects with vibration Loading, Pulling, and Pushing Tasks |
| Anterior and posterior spinocerebellar tract | Musculo-articular unconscious sensation | Cerebral cortex | Working on unstable surfaces that require postural adjustments against gravitational force |
The brainstem has an important sensory and motor function and acts as a primary distributor for somatosensory information from the spinal cord and the motor pathways of the cerebral cortex. The movements produced by the brainstem involve the entire body and are important for walking, eating, drinking, swimming, grooming, and sexual behavior13. The central part of the brainstem contains the nuclei of the cranial nerves and bundles of sensory and motor fibers. It is summarize the cranial nerves that related to the brainstem and their function; this, to show the relationship they have to produce action (praxis) and our proposal for neuropsychological rehabilitation of praxis (Table 2).
Table 2 Functions and quality of the cranial nerves and proposal for neuropsychological rehabilitation in somatodyspraxia
| Cranial nerves | Function | Quality | Proposal for neuropsychological rehabilitation of somatodyspraxia |
|---|---|---|---|
| Arousal/consciousness | Reticular formation | Activities to improve alertness Oculomotor nerve (III) Trochlear nerve (IV) |
|
| Abducens nerve (VI) | Motor function, involved in the muscular control of the eyes | Motor | Visual tracking activities |
| Facial nerve (VII) | Facial expression, gland discharge and taste sensation | Sensitive | Facial muscle massage Somatosensory stimuli in the facial oral tract Orofacial movements |
| Trigeminal nerve (V) | Sensitivity of the face, nose, teeth, jaw movements | Mixed | Tongue proprioceptive exercises, such as pushing to the front, to the sides, pulling the tongue, or carrying an object, like a pencil |
| Vestibulocochlear nerve (VIII) | Vestibular Information | Sensitive | Exercises, such as going up a ramp, going down, head down to pick up balls |
| Glossopharyngeal nerve (IX) | Sensitivity and movement of the tongue and pharynx | Mixed | Stimulate the inside of the cheeks and gums with cotton swabs, tongue depressors, teethers, or vibration brushes |
| Vagus nerve (X) | Sensory and motor actions of organs, such as the heart, blood vessels, and viscera | Mixed | Cardiovascular exercises that favor inhalation and
expiration Accessory nerve (XI) |
| Hypoglossal nerve (XII) | Control of neck and tongue muscles | Motor | Stimulate muscle tone of the neck with proprioceptive
exercises Swallowing exercises |
The cerebellum is involved in the synergy of movement through the spinocerebellar, vestibulocerebellar, and cerebrocerebellar bundles, by means of which movements are grouped correctly for the execution of acts that require special adjustments to establish and maintain balance and to regulate muscle tone, necessary for praxis. Our proposal for the rehabilitation of somatodyspraxia recommends working on the control of static and dynamic balance with the use of balance boards, balls and unstable surfaces. In addition, we suggest working on regulating motor rhythm, through activities for motor coordination of hands, fingers and feet. The most relevant pathways that reach the cerebellum and their function are shown, to specify the relationship they maintain with the production of action (praxis) and our proposal for neuropsychological rehabilitation of praxis (Table 3).
Table 3 Functions and fiber connections of the thalamic nuclei and proposal for neuropsychological rehabilitation in somatodyspraxia
| Thalamic Nuclei | Function | Fiber connections | Proposal for neuropsychological rehabilitation of somatoapraxia |
|---|---|---|---|
| Anterior | Emotional tone, recent memory mechanisms | Mamillothalamic tract, cingulate gyrus, hypothalamus | Use stimuli that have emotional value |
| Dorsomedial | Integration of somatic, visceral and olfactory information, and relationship with emotional sensations | Prefrontal cortex, hypothalamus, other thalamic nuclei | Proprioceptive stimuli that stimulate interoception |
| Dorsolateral, posterolateral, pulvinar | Unknown | Cerebral cortex, other thalamic nuclei | |
| Ventral anterior | Influences the activity of the motor cortex | Reticular formation, substantia nigra, striatum, premotor cortex, other thalamic nuclei | Motor activities that stimulate body schema |
| Ventral lateral | Influences the motor activity of the motor cortex | Reticular formation, substantia nigra, striatum, premotor cortex, other nuclei of the thalamus, cerebellum, and red nucleus | Apply motor activities that seek the integration of vestibular, visual and proprioceptive senses |
| Ventral posteromedial (VPM) | Relay for common sensations toward consciousness | Trigeminal lemniscus, taste fibers | Promote the recognition and expression of the sensations experienced with the material |
| Ventral posterolateral (VPL) | Relay for common sensations towards consciousness | Medial and spinal lemniscus | Promote the recognition and expression of the sensations experienced with the material |
| Intralaminar | Influences states of consciousness and alertness | Reticular formation, spinothalamic and trigeminothalamic tracts | Vertical vestibular, like jumping and hopping |
| Midline | Unknown | Reticular formation | |
| Reticular | The cortex regulates the thalamus | Cerebral cortex, reticular formation | Stimulate the state of attention |
| Medial geniculate body | Hearing | Inferior colliculus | Apply auditory stimuli during the integrated activity |
| Lateral geniculate body | Optic tract |
Sensory information from the brainstem (somatosensory, auditory, vestibular, and taste) reaches the thalamus and information from visual and olfactory pathways is added. The thalamus is associated with the integration of somatosensory information (touch, pressure, pain, and temperature), which is processed in the ventral posteromedial nuclei (head and neck) and ventral posterolateral nuclei (body). Then, it is transmitted to the somatosensory cortex of the parietal lobe. Auditory information is processed in the medial geniculate nuclei and sent to the auditory cortex in the temporal lobe. Vestibular information reaches the posterior ventral nuclei and is directed to the somatosensory cortex of the parietal lobe. The posteromedial ventral nuclei receive information from the sense of taste, which is directed to the gustatory area in the parietal lobe. Visual information reaches the lateral geniculate nuclei and is subsequently projected into the visual cortex in the occipital lobe. The olfactory information is received in the central part of the dorsal medial nuclei, and then it is projected toward the orbitofrontal cortex. For the rehabilitation of somatopraxis, according to our model, we suggest that the subject performs an activity that integrates proprioceptive stimuli of the head and body, vestibular, visual, and auditory. For example, standing on a balance board that forces him to make postural adjustments against gravitational force; throwing bags of different weights, textures, and shapes into a container, to see where the stimulus is directed and to listen when it falls, all integrated into a single action (praxis)48. The most relevant nuclei of the thalamus and their functions are shown, as well as their relationship with the production of action (praxis) and our proposal for neuropsychological rehabilitation of praxis (Table 4).
Table 4 Areas and functions of the cerebellum and proposal for neuropsychological rehabilitation in somatodyspraxia
| Area | Function | Fiber connections | Terminate in | Proposal for neuropsychological rehabilitation of somatodyspraxia |
|---|---|---|---|---|
| Spinocerebellum | Regulation of face and hand movements
Coordination of extremities (hands, fingers, and feet). |
Medial (vermis) | Dorsal nucleus | Motor activities using balance boards, balls and swings |
| Vestibulocerebellum | Control of static and dynamic balance Eye movement |
Vermian regions, flocculonodular lobe, fastigial
nucleus Anterior spinothalamic tract |
Spinal cord and motor nuclei | Visual tracking activities favoring the vestibular system |
| Cerebrocerebellum | Learning and maintenance of motor skills Error correction Rhythm regulation |
Cerebral cortex Ventral lateral nucleus of the thalamus |
Lateral areas of the contralateral hemisphere | Encourage adaptive responses |
The basal nuclei are important in motor learning39. They play an important role in inhibiting unwanted movements and promoting the desired ones, initiating the production of movements directed toward a goal, and modulating the force of the movements13 necessary for the execution of praxis. For neuropsychological rehabilitation of somatopraxis, according to our model, we suggest performing activities to inhibit movement, processing speed, and modulation of movement. Shown, are the basal nuclei, as well as their functions and the relationship they maintain with the production of action (praxis), as well as our proposal for neuropsychological rehabilitation of praxis (Table 5).
Table 5 Functions and pathways of the Basal nuclei and proposal for neuropsychological rehabilitation in somatodyspraxia
| Area | Function | Pathways | Fiber connections | Terminate in | Proposal for neuropsychological rehabilitation of somatodyspraxia |
|---|---|---|---|---|---|
| Caudate nucleus | Static and dynamic balance control Eye movement |
Medial (vermis) | Ipsilateral spinal cord | Visual tracking activities favoring the vestibular system | |
| Putamen | Generation of speed and range of motion Action control, motivation and cognition |
Vermian regions, flocculonodular lobe, fastigial
nucleus Anterior spinothalamic tract |
Vestibular nucleus | Spinal cord and motor nuclei | Throw balls and sacks, following a trajectory |
| Globus pallidus | Motor control and coordination | Excitatory-inhibitory | Caudate nucleus and putamen | Motor and prefrontal cortex | Promote the inhibition of motor stimuli upon verbal command |
Regarding the cortical areas involved, the parietal lobe comprises a multiplicity of areas and can vary in different parts of the parietal cortex. The most important are involved with the trajectory of movement of various parts of the body, orientation of the extremities, the control of body parts, the identification, size, and shape of objects, as well as the location and orientation of the object. For the rehabilitation of somatopraxis, we propose using objects of different textures and sizes to stimulate different parts of the patient’s body. We also suggest using objects with different temperatures, for example ice to stimulate cold, and others that stimulate pain. In addition, in our neuropsychological rehabilitation model, we propose carrying out activities that stimulate the internal representation of the body or body schema, such as jumping around, rolling your body on the floor or on a large cushion. Activities that require pulling, loading, and pushing, for proprioceptive stimulation, should also be included, for example, moving therapy equipment, huge cushions, and/or supporting your own weight from a trapeze48.
As mentioned above, praxis is an integrative function, in which the harmonious work of different neural systems promotes learning and maturation of each of the aspects involved in the production of voluntary and involuntary movement. The execution of praxis must be analyzed as a functional system, which requires the communication of the cortex, subcortex, and spinal cord. The integrated system forms a neural network involved in different actions, which when suffering selective damage through a pathological process can produce Somatopraxis.
Proposal of a neuropsychological rehabilitation model for apraxia
Based on the definition of somatodyspraxia, alterations in the processing of somatosensory and proprioceptive information lead to difficulties in understanding the body schema and the relationship of the body itself with respect to objects, making postural adjustments for the correct execution of movements, and therefore carrying out many of the activities of daily living, such as dressing, eating, or combing hair.
In this article, we propose that somatodyspraxia is the primary factor of apraxia and we present a neuropsychological intervention model for Apraxia and Somatodyspraxia that focuses on the stimulation of the somatosensory and proprioceptive systems, body schema, and postural adjustments.
The intervention program in this approach is based on a neuroanatomical and neurophysiological theory. We recommend focusing on the stimulation the proprioceptive and somatosensory systems, because this specific information, after traveling through the somatosensory pathway and the thalamus, reaches parietal areas which communicate with primary motor areas. The integration of this information in the CNS helps regulate the production of adequate motor responses for performing praxis. In addition to stimulating the somatosensory and proprioceptive systems, the patient’s movement is guided and regulated through language.
By stimulating the somatosensory system with touch, pain, pressure, vibration, and temperature stimuli, one favors the understanding of the body schema, which is necessary for the execution of movements such as writing, dressing, eating and swallowing, the articulation of words or sphincter control. Muscle and joint activation by stimulating mouth and phonation apparatus movements favor for proprioception. Intervention through the vestibular system and management of postural adjustments is necessary for the adequate head and eyes movements for drawing, the integration of two-dimensional and three-dimensional visual stimuli, walking, handling objects, and even getting dressed.
In this section, we propose a neuropsychological rehabilitation model for apraxia, based on the PAINT Model. Our model proposes conducting a comprehensive rehabilitation program based on the stimulation of the somatosensory and proprioceptive systems, body schema and postural adjustments involved for the execution of praxis, as part of a complex functional system45 (Fig. 2).
One of the main objectives of our PAINT Neuropsychological Rehabilitation Model is to stimulate the somatosensory system through all its functions: pain, touch, temperature, pressure, texture, and proprioception, since the nervous system requires somatosensory feedback to control voluntary movement. We base this on the fact that, during voluntary movement, the CNS is continuously receiving information from both, somatosensory signals derived from changes in the external environment and from the person’s postural changes46. The integration of somatosensory information is critical for motor control and movement recognition, and the correct performance of movements that involve manipulating objects and purposive actions.
Another of the main objectives of our PAINT Neuropsychological Rehabilitation Model is to stimulate the proprioceptive system, and perception or awareness of the position and movement of body. Proprioception is the body´s self-awareness that tells the body where it is in space. We receive proprioceptive input from our sensory receptors located in muscles, tendons, joints and internal organs47, and it is crucil to the brain, as it plays a large role in posture, body awareness, use of tools and purposive actions. This information is processed in the somatosensory areas of the posterior parietal cortex. We propose activities that stimulate proprioceptive senses, such as pushing and pulling with the patient´s own weight, pulling a rope or heavy mat; or resistance exercises that involve different body segments, including the tongue48.
The PAINT Neuropsychological Rehabilitation Model for apraxia also pursues the stimulation of the body schema and postural adjustments of the subject. The goal is to promote mobility of the upper and lower limbs, as well as the use of both hands and feet in praxis. This model for apraxia proposes stimulation of the body schema by providing tactile-kinesthetic stimuli to the upper and lower limbs. The therapist guides the movements by placing him or herself behind the patient, so that the actions are natural, while simultaneously giving a verbal description of the movements, thus favoring the correct connection between the movement and its execution with the use of objects. Regarding the problems of managing the body in space, we seek to work on understanding the place the body occupies in space in relation to an object.
Our PAINT Neuropsychological Rehabilitation model aims to stimulate postural adjustments by making different movements with the body or putting it in awkward positions from which patients must move several body segments such as the arm, leg or, even the head. This allows the stimulation of postural adjustments against gravitational force, through movements of the head and body on surfaces such as balls, seesaws or suspended equipment, with the goal of causing linear accelerations and angular accelerations. Our Rehabilitation Model seeks to integrate proprioceptive and visual information, to establish diagrams of the position and dynamics of body movements, the verticality within the three-dimensional gravitational field49, the position of the head with respect to gravity and the detection of changes in its movement45, as well as determining speed and direction of the movements.
By understanding the concept of somatodyspraxia, one can exploit it during patient rehabilitation after acquired brain injury. The rehabilitation of the different propioceptive and somatosensory components allows the patient to recover vital neurological pathways for the re-establishment of daily life activities. Our proposed term of somatodyspraxia and its importance in the PAINT Neuropsychological Rehabilitation model will allow medical and rehabilitation personnel to have a better understanding of the neurological and neuropsychological concepts that ultimately affect a patients quality of life.
Conclusion
Acquired brain injury commonly causes alterations in different cognitive functions. Adults with brain injury can present a form of apraxia, which we have called somatodyspraxia; an alteration in somatosensory and proprioceptive processing, after acquired brain injury, which makes it difficult to manage the body schema and the postural adjustments necessary for the execution of tasks or actions. Patients with these types of alterations have difficulty in carrying out activities of daily life, which impacts their quality of life. The precise recognition of somatodyspraxia in adults is relevant because it can guide the establishment of a rehabilitation program focused on the stimulation of the somatosensory system, proprioceptive system, body schema, and postural adjustments.











 nueva página del texto (beta)
nueva página del texto (beta)