Introduction
Anxiety and depression are the most widespread mental disorders, most prevalent in low- and middle-income countries — whose rate remains increasing in the world population due to social, economic, physical, and patient context factors1. This situation will generate more significant chain conflicts, and if the global burden of mental illness is considered, it will imply that contemporary society will eventually begin to need not only a higher amount of clinical alternatives to address these pathologies but more effective methods and techniques to treat them.
Then, clinical models for treating anxiety problems should consider this phenomenon from a transdisciplinary point of view, not to replace traditional intervention models, but to add the participation of neurosciences together with the use of technological tools2.
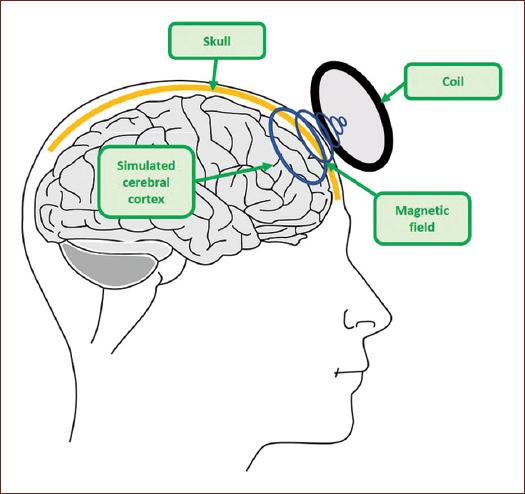
Transcranial magnetic stimulation (TMS) is an unorthodox and avant-garde treatment for clinical intervention3. Compared with traditional psychopharmacology, which aims to treat neurobiological mechanisms, TMS generates a stimulation directly in the functioning of any region of the cerebral cortex, both in inhibitory and excitatory neuronal circuits3. On the other hand, TMS affects neurophysiological processes and neurobiochemicals, without being invasive like some other procedures4, since it favors the depolarization of the membrane of neurons (Fig. 1) by generating a sufficient electromagnetic field to trigger action potentials5.
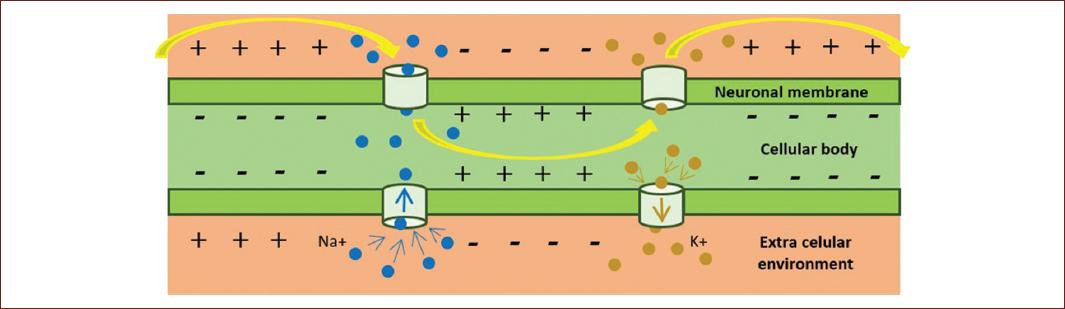
Figure 1 Depolarization is the process that allows the transmission of the nerve impulse when there is a change of charge between the outside of the membrane, from positive to negative, and the inside, from negative to positive. This process enables the transmission of the nerve impulse and, therefore, of the neuronal intercommunication.
Compared to direct electrical stimulation, TMS allows stimulation to act more focally4. It has been considered that simple magnetic pulses or “trains” (pulse bursts) are capable of depolarizing the membrane of a group of neurons, either from some axon or some dendritic feet, initiating an exciting, or inhibiting chain reaction3.
The theoretical principles of magnetic induction proposed by Michael Faraday toward 1831 are the basis of the TMS. However, until 1984, Anthony Barker et al. managed, through extensive research, to develop a neurostimulator that could generate depolarization of cortical neurons, causing movements3. Eventually, the development of the stimulation technique gained its characteristics and stimulation parameters to turn it into what is currently known as the TMS.
TMS is a technique with a mechanism of action that consists in the application of a magnetic field (magnetic pulses) of defined intensity which is produced by a coil going through not only the skull but also the scalp of a person to reach the cerebral cortex, where it will affect, inhibiting or exciting, and neuronal function (Fig. 2)6.
Practically, for a magnetic neurostimulator to produce a magnetic field that is capable of stimulating cortical neurons, it must use an electric current intensity of 7-10 kA, which is produced by an energy capacitor, a charging circuit, and one of discharge, as well as with an electronic switch that flows through a coil up to 500 J in the form of a pulse of approximately 1-ms duration7.
This treatment has been well received by multiple international clinical institutions as a “non-experimental” medical treatment for psychiatric conditions, especially for the effective treatment for major depression and promising usefulness for social anxiety treatment8. Related to panic disorder, a study conducted by Dresler in 2009 reported a case in which TMS was able to modulate cortical functions during an emotional crisis, that is, a panic attack9.
The objectives of this review were to analyze how effective the TMS intervention has been found in anxiety disorders — according to the characteristics of the samples and the experimental designs — and to determine the implications for future interventions based on the PRISMA criteria10.
Anxiety disorders
Anxiety is an emotional state in which humans naturally express to certain environmental stimuli. Thus, anxiety, understood as a physiological chain reaction activated by the autonomic nervous system, alerts individuals to dangerous situations that arise in the surroundings manifesting itself as adaptive defensive behavior that allows human survival.
Methodology
A specific search was conducted in the scientific research repositories such as PUBMED, Neurology, Medline, Elsevier, and others that meet international criteria until 2020. The first search was performed using the following keywords:
Panic disorder AND Magnetic transcranial stimulation OR Repetitive magnetic transcranial stimulation OR TMS, Generalized Anxiety Disorder AND symptoms OR state, Social Anxiety Disorder OR specific phobia OR depression AND anxiety, depersonalization disorder OR Parkinson AND depression AND anxiety. These results were included by virtue that it could be analyzed how efficient repeated TMS (rTMS) was for anxiety symptoms.
Various studies were selected according to the following inclusion criteria presented in Table 1.
Table 1 Inclusion and exclusion criteria for literature search
| Inclusion criteria | Exclusion criteria |
|---|---|
| Date of recent publication | Date of non-recent publication |
| Date of publication not recent that provide information to contextualize the subject or lay historical foundations of the topics | Date of non-recent publication and obsolete information |
| TMS is applied to some anxiety disorder | TMS was not applied to any psychiatric disorder |
| Explains the TMS application protocol | |
| Explains the consequences of the application of TMS |
During the systematic review, research was found, beyond anxiety itself, highlighting emotional processing and, in some cases, using a technique with the same physical principle as TMS, called Intermittent Tetha Burst Stimulation (iTBS) or Intermittent Stimulation of Theta bursts. Unlike rTMS, which application varies from values below 1 Hz to 50 Hz, iTBS provides 10 bursts of three biphasic pulses of 100 ms at 50 Hz repeated at 200ms intervals, that is, 5Hz at theta frequency11.
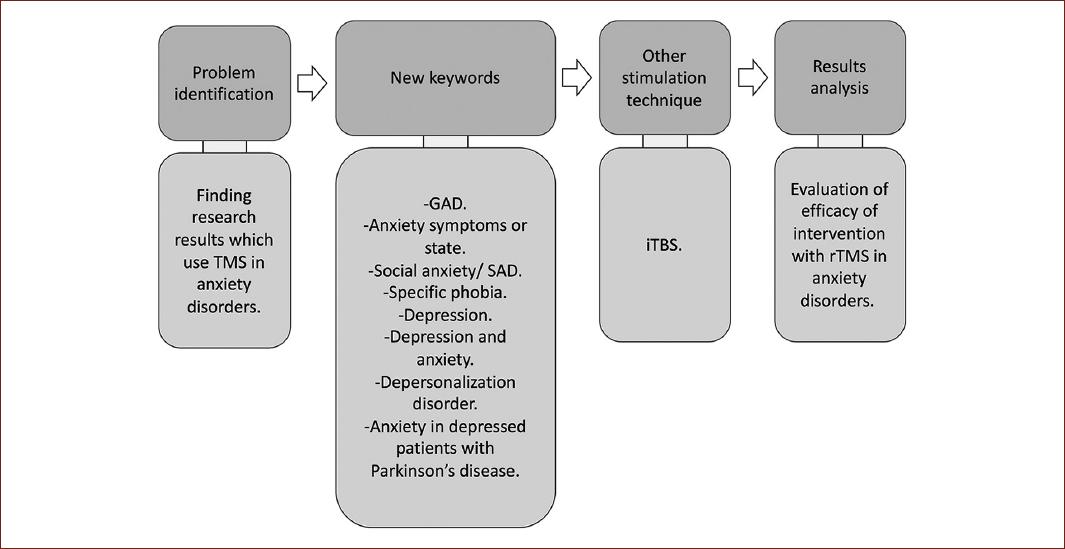
Selected documents were classified according to the information and type of research. Thus, the literature search was performed as presented in Fig. 3. The information extracted from the articles was organized to recognize the characteristics of the sample, experimental design, inclusion and exclusion criteria, the method and instrument of intervention, adverse symptoms, and results. The articles were rated according to the guidelines of the PEDro Scale12.
Results
The papers selected for the critical analysis of this systematic review are presented in Table 1. It is worth noting that the intervention method and the results were highlighted since this allows determining whether rTMS is emerging as a useful technique for anxiety disorder treatment and other disorders that also present anxiety symptoms.
Discussion
It was found that TMS, applied as a non-invasive intervention technique, is effective for different psychiatric disorders. Although the results of rTMS have been mostly studied in depression, it has been found that enough research has also been done in other pathologies such as those of anxiety.
The results have been organized and classified according to the effectiveness of treatment through rTMS, according to data within table 2.
Table 2 Organization of investigations according to effectiveness results
| Disorder/mental process | Effective treatment | Non-effective treatment |
|---|---|---|
| Disorder of panic/anguish | García-Toro et al.14; | Deppermann et al.43; |
| Machado et al.42; | Zwanzger et al.41 | |
| Mantovani et al.39 | ||
| Disorder of general anxiety | Diefenbach et al.13 | |
| Anxiety/symptoms/state of anxiety/fear and anxiety | Balconi and Ferrari19,20,26 | Baeken et al.24; |
| Machado et al.15; | Vanderhasselt et al.25 | |
| Vanderhasselt et al.23; | ||
| Zwanzger et al.28 | ||
| Anxiety/social phobia | Pallanti et al.36; | |
| Balderson et al.45 | ||
| Specific phobia | Deppermann et al.29; | Deppermann et al.33; |
| Herrmann30 | Notzon et al.34 | |
| Anxious depression/depression and anxiety | Diefenbach et al.18; | |
| LaSalle-Ricci et al.21 | ||
| Depression | Deppermann et al.29; | |
| Fitzgerald et al.27 | ||
| Depersonalization | Jay et al.16 | |
| Parkinson, anxiety and depression | Kormos22 | |
| Automatic emotional reactions/emotional processing | Berger et al.31; | Vennewald et al.32 |
| De Raedt et al.38 |
Studied disorders
According to the 34 studies collected (33 = 100%, considering the references Machado et al.15 and Paes et al.17 report two investigations), 66.66% were carried out with some specific disorder or symptoms of anxiety (23 = 100%). Therefore, 35.29% were performed with panic disorder, 17.64% were performed with phobic disorders, and 47.05% were performed with anxiety symptoms or some unspecified disorder of anxiety.
Sample description
Regarding the sample size, 36.36% of the studies were conducted with 30 or more participants. Samples of 67 and 40 patients were used for the disorder of panic, other samples of 25 and 30 patients were used for anxiety symptoms or some disorder of anxiety not specified and, in the case of phobias, all the samples were between 41 and 30 patients.
In general, these studies were carried out in hospitals. On the other hand, 64.70% were performed with very variable samples from < 30 patients. For example, in generalized anxiety, it was found that an investigation used a sample of 25 patients and other 10. There was more variability for the panic disorder since small samples were found between three and 15 volunteers; the same happened for symptoms or some unspecified disorder of anxiety, where samples were presented in the range of 10 to 28 participants.
Table 3 describes some other attributes of samples specified in the papers found during the systematic search.
Criteria for inclusion and exclusion of studies
Due to the inclusion and exclusion criteria, roughly, subjects had to be diagnosed according to specific scales with the study pathology, and in counterpart, not suffering any other disease of the central nervous system, psychiatric or neurological, or cardiovascular; only the concomitance of some other disease was allowed in Mantovani et al.39 and Mantovani et al.40 In none of the cases, the selection of volunteers was made according to gender.
Intervention protocol and treatment efficiency
Intervention protocol for anxiety disorders prevailing was 1 Hz with an intensity between 90 and 110% of the motor threshold, and it is essential to note that there is a correlation between stimulation characteristics and its results. Notably, in Zwanzger et al.41, treatment was carried out at a low frequency, but it was not performed repeatedly, and the results were not as expected. Regarding the results of Deppermann et al.43, no strong results were found since the registered psychophysiological arousal may be due to the tasks performed by those evaluated and not properly to the effects of stimulation; the results of Vanderhasselt et al.23 and Baeken et al.24 had no favorable effects because the stimulation was performed with high frequencies (10 Hz) as the frequency approved for depression.
Results presented in Deppermann et al.33 are inconclusive by virtue that it cannot be specified how stimulation modulates neuronal activation. On the other hand, Notzon34 stated that a single stimulation session is not enough to generate effects on phobic symptoms, and mainly the frequency of the protocol was not presented. The results presented in Balconi19,20 were favorable, although protocols with an intermediate intensity (5 Hz) were applied. In other words, 72.72% of the sources consulted showed favorable results after using the rTMS, while 27.27% indicated non-favorable or conclusive results, although it should be noted that the protocol is not the same in all cases. In this context, 68.18% stimulated the right dorsolateral prefrontal cortex, demonstrating that the efficacy of rTMS treatment for these types of disorders is achieved when this neuroanatomic region is stimulated, because it reduces hypermetabolism and neuronal hyperexcitability.
Neurostimulation equipment used for rTMS
The most used neurostimulator, as reported in the documents was Magstim18,19,22,23,25,42, followed by Dantec MagPRo14,28-30, and only one report13 used Neuronetics XPLOR, and other one MagVenture MagPro 10045.
Adverse reactions reported
In the present review, Baeken et al.24 reported a secondary dermatological reaction; García-Toro et al.14, a patient who reported mild and transient headache, and Diefenbach et al.13 reported that one patient suffered pain at the stimulation site. Although the administration of this neurostimulation technique is endorsed by the Food and Drug Administration (FDA), results indicate that it is favorable, it has been indicated that research on the effects should continue. It is important to mention that research using functional neuronal evaluation tools in which clinical factors can be ruled out to demonstrate the effects of magnetic stimulation is needed.
In two studies, there was a control group analysis with a placebo effect13,43, and it was possible to show that magnetic stimulation was effective in Diefenbach et al.13, which is not in Deppermann’s43.
Limitations of this review
Although conventional intervention methods, which include psychotherapy and pharmacology, have proven to be effective because both have been studied for a long time, protocols need to conclude whether magnetic stimulation effects are effective or not. About the above, it should be considered that subjective variables, that is, the references of the patients about their perception of improvement, do not allow a more objective analysis, and it is necessary to carry out the evaluation of the effect with functional and structural evaluation tools of the stimulated cortical areas.
On the other hand, it is worth noting that no studies were found in which rTMS has been used during a panic attack so that its effect on physiological or other variables could be understood in greater depth. In the same way, this systematic review of literature aimed to determine how effective rTMS is in anxiety disorders, highlighting the importance of continuing the research on the topic.
Regarding to the PEDro scale, one article fulfilled 100% of the criteria, six with 90.90%, three with 27.27%, one with 72.72%, one with 63.63%, and four with 54.54%, while the rest of the works were found below this last percentage, so it could be said that half of the papers reported met at least more than half of the criteria established in the scale PEDro. In this regard, it is necessary for scientific research to demonstrate the usefulness of any clinical intervention, in this case the rTMS in anxiety disorders, and expose the risks of bias, either due to the characteristics of the samples or the non-contemplation of certain variables, as well as to break down their design, their procedures and results explicitly so that other researchers clearly know the benefits.
Conclusions
rTMS favors neuromodulation through the generation of action potentials6, which facilitates the treatment of pathologies related to emotional components, such as anxiety.
The most effective protocol to treat anxiety disorders with the reported rTMS uses low-frequency stimulation (1 Hz), with 110% of the motor threshold, applied on the right dorsolateral prefrontal cortex with a 30-min train 5 times a week, for a month.
Finally, the use of rTMS could favor efficacy of psychotherapeutic procedures since these are understood as methods favoring learning, and neurostimulation promotes neuroplasticity.











 nueva página del texto (beta)
nueva página del texto (beta)