COVID-19. Neurological and neuropsychiatric manifestations and their neuropathology
The most frequently reported neurological manifestations in patients infected with the SARS-CoV-2 coronavirus are headache (6%-15%), anosmia (41.0%), and ageusia (38.2%). The headache is described as generalized, hemicranial, or occipital of the oppressive type and which increases with physical activity or head movements, characteristics suggesting valsalva effect and therefore cerebrospinal fluid flow dysfunction and periarterial and perivenous cerebral glymphatic system dysfunction1-3
Deterioration of consciousness has been reported in up to 14.8% of cases of COVID-19 complicated with ARDS or Multiple Organ Dysfunction in the report by Mao et al., or as agitation in 69% of the cases and confusion in 45% of patients with post-intubation ARDS. Symptomatology that could correspond to delirium due to multiple causes: sedative drug effects, intubation with prolonged assisted ventilation, hypoxic, or metabolic encephalopathy4,5. Ischemic cerebrovascular disease has been reported in between 2.8% and 16.7%, encephalopathy with epileptic seizures 0.5%.6 Five cases of Guillain-Barre syndrome were reported among 1200 cases of patients with COVID-19 in Italy7. In the United Kingdom out of 125 patients: cerebrovascular disease 57 (45.6%) ischemic, 9 (7.2%) hemorrhagic, 9 (7.2%) unspecified encephalopathy, 7 (5.6%) encephalitis, 10 (8%) psychosis, 6 (4.8%) neurocognitive disorder, and 4 (3.2%) affective disorder8.
The presence of vasculitis/endotheliitis of small vessels with microhemorrhages and microinfarcts without damage to the large supra-aortic or intracerebral vessels has been reported in some cases (Figs. 1 and 2)9. However, two meta-analysis studies by stroke and COVID-19 report that elderly patients with elevated levels of D-dimer are associated with occlusion of the great vessels and increased mortality rates10,11.

Figure 1 A: 3D coronal reconstruction of supra-aortic and intracerebral vessels B: T2 brain MRI with microhemorrhages in both pale globe.

Figure 2 Axial (A, B, and C) and coronal (D) Flair MRI. Diffuse hyperintense images suggestive of ischemic lesions in basal ganglia and cerebellar peduncles.
It is not yet clear whether SARS-CoV-2 is neurotropic in humans. Viral neuroinvasion could be achieved in a variety of ways, including trans-synaptic transfer through infected neurons, entry through the olfactory nerve, by the cribriform plate of the ethmoid bone to the glymphatic system with infection of astrocytes, infection of the vascular endothelium, or migration of leukocytes across the blood-brain barrier with manifestations of the central or peripheral nervous system (Table 1)12-14.
Table 1 Neuropathogenesis and neurologic manifestations of the central or peripheral nervous system in positive COVID-19 patients
| Clinical entity | Signs and symptoms | Laboratory and cabinet | Pathogenesis |
|---|---|---|---|
| Encephalopathy | Altered mental state | MRI: Non-specific EEG: Diffuse slowing CSF: Normal CSF SARS-CoV-2 RT-PCR: Negative | Multiple Organ Dysfunction. Hypoxemia. Systemic inflammation. Endotheliitis |
| Encephalitis | Altered mental status and Central Nervous System Dysfunction | MRI: Non-specific EEG: Diffuse and focal Slowing CSF: Abnormal Pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Negative | Inflammation of the Central Nervous System |
| Viral encephalitis | Fever, altered mental status and Central Nervous System Dysfunction | MRI: Focal or multiple abnormalities EEG: Diffuse and focal Slowing CSF: Abnormal pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Positive Brain tissue: Positive Antigen or RNA | Viral invasion to the brain parenchyma |
| Viral meningitis | Fever, Headache with stiff neck, Kernig/Brudzinski positive | MRI: Non-specific EEG: Normal or focal abnormal CSF: Abnormal Pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Positive | Subarachnoid viral invasion |
| Anosmia and/or Augesia | Loss of smell/loss of taste | Clinical tests for assessing smell and taste: Abnormal | Viral invasion, peripheral or central? |
| Cerebrovascular disease | Focal motor or sensory neurological deficit | MRI: Ischemia or hemorrhage Laboratory: Increased markers of inflammation and abnormal coagulation factors | Coagulopathy |
| Acute disseminated encephalomyelitis | Headache, disorientation, acute neurological deficit with psychiatric manifestations | MRI: Hyper intensive lesions in Flair with supratentorial and subcortical predominance. CSF: Normal or with ↑ Proteins | Viral post-infection |
| Guillain-Barre syndrome | Ascending symmetrical flaccid muscle weakness with areflexia and pain | CSF: Cells 0-5 (Normal) + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Negative. Conduction velocity and electromyography: Abnormal | Viral post-infection |
| Muscular lesion | Myalgia | CPK: Elevated | Myopathy or Myositis? |
| Encephalopathy | Altered mental state | MRI: Non-specific EEG: Diffuse slowing CSF: Normal CSF SARS-CoV-2 RT-PCR: Negative | Multiple organ dysfunction. Hypoxemia. Systemic inflammation. Endotheliitis |
| Encephalitis | Altered mental status and Central Nervous System Dysfunction | MRI: Non-specific EEG: Diffuse and focal slowing CSF: Abnormal Pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Negative | Inflammation of the Central Nervous System. |
| Viral encephalitis | Fever, altered mental status and Central Nervous System Dysfunction | MRI: Focal or multiple abnormalities EEG: Diffuse and focal slowing CSF: Abnormal pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Positive Brain tissue: Positive antigen or RNA | Viral invasion to the brain parenchyma |
| Viral meningitis | Fever, Headache with stiff neck, Kernig/Brudzinski positive | MRI: Non-specific EEG: Normal or focal abnormal CSF: Abnormal pleocytosis + + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Positive | Subarachnoid viral invasion |
| Anosmia and/or Augesia | Loss of smell/loss of taste. | Clinical tests for assessing smell and taste: Abnormal | Viral invasion, peripheral or central? |
| Cerebrovascular disease | Focal motor or sensory neurological deficit | MRI: Ischemia or hemorrhage Laboratory: Increased markers of inflammation and abnormal coagulation factors | Coagulopathy |
| Acute disseminated encephalomyelitis | Headache, disorientation, acute neurological deficit with psychiatric manifestations | MRI: Hyper intensive lesions in Flair with supratentorial and subcortical predominance. CSF: Normal or with ↑ Proteins | Viral post-infection |
| Guillain-Barre syndrome | Ascending symmetrical flaccid muscle weakness with areflexia and pain | CSF: Cells 0-5 (Normal) + ↑ Proteins CSF SARS-CoV-2 RT-PCR: Negative. Conduction velocity and electromyography: Abnormal | Viral post-infection |
| Muscular lesion | Myalgia | CPK: Elevated | Myopathy or Myositis? |
Incidence and mortality, by age and gender, of COVID-19
According to the information provided by the WHO mission in China, from the first 41 cases reported between December 8, 2019, and January 2, 2020, it extended to 86,889 confirmed cases as of July 28, 2020, and 89,827 COVID-19 cases as of August 28, 2020. The median was 51 years of age with a majority of cases (77.8%) between 30 and 69 years of age, 51% of these cases were male. On May 28, 2020, a worldwide pandemic was reported with 5,808,946 and by June 28, 10,115,912 with 501,206 deaths, with a 6.4% fatality rate. On 28 July 2020, 16,662,462 positive cases were reported with 658,861 cumulative deaths and 24, 649, 431 COVID-19 cases as of August 28, with 835, 793 cumulative deaths. On September 28, 2020, were reported 33,034,598 with 996,342 deaths and by October 28 were 44,481,667 positive COVID-19 cases with 1,172,086 cumulative deaths. A global of 82,777,305 cases and 1,806,155 deaths were reported on December 31, 2020.
In the Americas, the crisis has not yet reached its critical point, with the United States being the most affected country in the region and also in the world, since as of July 28, 2020, there were 4,347,717 positive COVID-19 cases and 149,209 deaths reported, figure that increases to 11,715,316 positive cases for COVID-19 with 252,535 deaths as of November 20, 2020 (Table 2). By December 31, 2020, the number of accumulated positive cases rose to 19,744,734 and 342,395 people died15.
Table 2 COVID-19 Pandemic.
| Country | COVID-19 + Cases | Deaths | % | C/100,000 |
|---|---|---|---|---|
| United states of America | 11, 715, 316 | 252, 535 | 2.15 | 77.19 |
| India | 9, 004, 365 | 132, 162 | 1.46 | 9.77 |
| Brazil | 5, 981, 767 | 168, 061 | 2.80 | 80.23 |
| France | 2, 137, 096 | 47, 201 | 2.20 | 70.46 |
| Russia | 1, 998, 966 | 34, 525 | 1.72 | 23.90 |
| Spain | 1, 541, 574 | 42, 291 | 2.74 | 23.79 |
| United kingdom | 1, 456, 940 | 53, 870 | 3.69 | 81.02 |
| Argentina | 1, 349, 434 | 36, 532 | 2.70 | 82.10 |
| Italy | 1, 308, 528 | 47, 870 | 3.65 | 79.21 |
| Colombia | 1, 225, 490 | 34, 761 | 2.83 | 70.01 |
| Mexico | 1, 019, 543 | 100, 104 | 9.81 | 79.33 |
| China | 91, 935 | 4, 742 | 5.15 | 0.34 |
Global cases: 58, 014, 491. Global deaths: 1, 378, 866. Accumulated cases and deaths by country as of November 20, 2020.
In Mexico, the first case of a person with imported COVID-19 was reported on February 28, 2020. The Mexican Ministry of Health and CONACYT (National Council for Science and Technology) reported on May 28, 2020: 81,400 confirmed cases of COVID-19 with 9044 deaths and in 1 month, June 28, 2020, it almost tripled with 216,852 cases with 26,648 deaths. By July 28, 2020, there were 402,697 cases positive for COVID-19, 53.31% male and 46.69% female, of which 72.37% were outpatient and 27.63% were hospitalized, reporting 44,876 cumulative deaths, predominantly male. On August 28, 2020, 6 months after the first positive COVID-19 case in Mexico, were reported 585,738 confirmed cases, with a cumulative fatality of 63,146 people. On September 28 were reported 733,717 with 76,603 deaths, by October 28, 2020, were 906, 863 positive COVID-19 cases, with a cumulative deaths rate of 90,309. On 20 November 2020, 1,025,969 cases, 51% male and 48.94% female, with 100,823 deaths (Table 3). And as of December 31, 2020, the total accumulated number of positive cases reported was 1,426,094 and 125,807 deaths16.
Table 3 COVID-19 in Mexico. Cumulative cases and deaths from February 28, 2020 to November 20, 2020
| Ciudad de México | 184,636 | 16,770 | Sinaloa | 23,308 | 3,848 |
|---|---|---|---|---|---|
| Estado de México | 104,341 | 11,443 | Guerrero | 23,112 | 2,335 |
| Nuevo León | 61,545 | 4,384 | Yucatán | 22,931 | 2,005 |
| Guanajuato | 55,967 | 3,730 | Durango | 18,212 | 1,017 |
| Sonora | 41,118 | 3,340 | Querétaro | 17,804 | 1,369 |
| Veracruz | 38,549 | 5,147 | Hidalgo | 17,638 | 2,543 |
| Jalisco | 38288 | 4,568 | Quintana Róo | 13,992 | 1,885 |
| Coahuila | 38,279 | 3,029 | Zacatecas | 13,828 | 1,159 |
| Puebla | 38,215 | 5,037 | Baja California Sur | 13,684 | 650 |
| Tabasco | 36,075 | 3,100 | Aguascalientes | 11,217 | 1,007 |
| Tamaulipas | 34,014 | 2,917 | Tlaxcala | 8,872 | 1.193 |
| San Luis Potosí | 32,267 | 2,308 | Chiapas | 7,657 | 1,088 |
| Michoacán | 27,150 | 2,167 | Morelos | 7,404 | 1,271 |
| Chihuahua | 26,974 | 3,035 | Nayarit | 6,997 | 911 |
| Baja California | 24,840 | 4,098 | Colima | 6,963 | 785 |
| Oaxaca | 23,455 | 1,783 | Campeche | 6,673 | 901 |
| Total | 1,025,969 | 100,823 |
Risk factors for COVID-19 lethality
The risk factors increase the possibility of Acute Respiratory Distress Syndrome (ARDS) and death for patients who are infected with the new coronavirus SARS-Cov-2 are:
1. Age and Gender
The number of COVID-19 positive patients who are asymptomatic is unknown, with percentages ranging from 3% to 6%. Of the symptomatic patients, 26% have mild uncomplicated disease (Phase I), 65% have moderate to severe symptoms (Phase II), and only 9% have severe symptoms that are complicated by pneumonia that progresses to ARDS or Multiple Organ Dysfunction (Phase III) (Table 4).
Table 4 Severity Levels and Evolution of COVID-19
| Severity levels and evolution | Clinical, laboratory and radiological findings |
|---|---|
| Phase I - Uncomplicated disease |
Fever, rhinorrhea, odynophagia, cough, myalgia, and headache |
| Phase II or pulmonary phase - Mild pneumonia |
Confirmed with chest X-ray or CT scan (CO-RADS 2-3). SaO2 >90%. RT-PCR + |
| - Severe pneumonia | Severe pneumonia Fever, productive cough, dyspnea. Chest CT scan (CO-RADS 4-5). SaO2 <90% and tachypnea ≥ 30/minute. RT-PCR + IgM +, IgG + Lymphopenia <0.8×109/L. Thrombocytopenia <100×109/L D-dimer elevation >1 mg/L. PCR elevation Ferritin elevation >300 mg/L IL-6 elevation >7.4 pg/mL Procalcitonin elevation ≥0.5 ng/mL |
| Phase III or hyper-inflammatory phase - Acute Respiratory Distress Syndrome (ARDS) |
Cough, dyspnea. Chest CT with bilateral
ground-glass opacities, with hypoxia: - Mild: 200 mmHg <PaO2/FiO2 ≤300 - Moderate: 100 mmHg <PaO2/FiO2 ≤200 - Severe: PaO2/FiO2 ≤100 mmHg |
| - Multiple Organ Dysfunction Syndrome by Septicemia | Organic dysfunction on the SOFA Score > 2 points or an acute change in the Quick Sofa with > 2 criteria |
| - Septic shock | Arterial hypotension that persists despite volume replacement with solutions and requires vasopressors to maintain MAP ≥65 mmHg and lactate ≥ 2 moll/L (18 mg/dL) in the absence of hypovolemia. |
In multiple logistic regression, the male sex was associated with severe symptoms (odds ratio [OR] 2 5 [IC 95% 1 1-6 1). The probability of severe symptoms increased slightly with age, although only people with 60-69 years of age had a significantly higher risk compared to the baseline category, people with 50-59 of age (OR 3 4 [95% 1 4-9 5). Males accounted for 63.7%17,18.
In Mexico, the reported case fatality as of June 28, 2020, represents 12.28% with 26,648 of the 216,852 positive COVID-19 cases. Males predominate with a 66% (17,569) versus a 34% (9,079) female. The age group of 92.65% is over 40 years of age. By July 28, 2020, the total number of cumulative deaths was 44,876, 64.94% male and 35.06% female. On August 28, 585,738 positive COVID-19 cases, 52.52% men, 47.49% women, were reported with 63,146 deaths, 64.43% men, 35.57% women, with a rate per 1000 cases of 38.24-44.05 between 70 and 99 years of age, compared to 0.92 in those under 29 years of age, 76,603 deaths by September 28, 90,309 cumulative deaths by October 28, 2020, and 100,823 on November 20, 2020, 63.74% male and 36.26% female (Fig. 3).

Figure 3 COVID-19 Mexico. Cumulative deaths by age and gender. Total: 100,823 February 28 to November 20, 2020.
2. Obesity, type 2 diabetes mellitus, and systemic arterial hypertension
An age of over 65 years and the male gender are risk factors for critical complication in patients infected with the new coronavirus SARS-CoV-2, but comorbidities also increase the risk of lethality in patients with C0VID-19. Richardson et al. reported in 5700 patients in New York City, the presence of Arterial Hypertension in 3026 (56.6%), Obesity with a Body Mass Index (BMI) greater than 35 in 1737 people (41.7%), Diabetes mellitus in 1808 (33.8%), and Sleep Apnea in only 154 patients (2.9%).19 Out of a group of 124 people in France with COVID-19, 85 patients (68.6%) required assisted mechanical ventilation (AMV), and the OR in cases requiring AMV with BMI > 35 kg/m2 versus patients with BMI <25 kg/m2 was 7.36 (95% CI 1.63-33.14) regardless of age, diabetes, or arterial hypertension20.
In Mexico, the Ministry of Health reports on November 20, 2020, that in 1,025,969 positive cases for C0VID-19, 51.06% were male and 48.94% female, arterial hypertension was in 20.09%, obesity 19.59%, diabetes mellitus 16.44%, and smoking 7.77%. In the critical group with the death of 100,823 people (9.82%), 63.74% were of the male gender and 36.26% of the female gender, arterial hypertension was present in 45.38%, diabetes mellitus in 38.58%, obesity in 23.90%, and smoking in 8.73% of the cases.
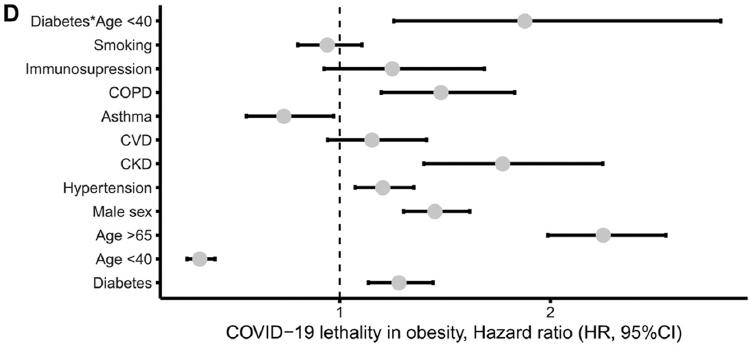
Bello-Chavolla et al. in a retrospective analysis of 15,529 SARS-Cov-2 positive patients compared to 46,960 SARS-Cov-2 negative persons found: obesity in 3,215 (20.7%) versus 6,570 (14%), respectively, arterial hypertension in 3,370 (21.7%) versus 7353 (15.7%), and diabetes mellitus in 2,831 (18.2%) versus 5,163 (11%). Considering that the coexistence of two comorbidities such as obesity and diabetes mellitus, particularly of early onset, increases the risk of severe complications in patients with COVID-19 (Fig. 4)21.
Treatment of COVID-19
The COVID-19 pandemic represents the largest global public health crisis of this generation and potentially since the outbreak of the pandemic influenza in 1918. The speed and volume of clinical trials launched to investigate possible therapies for COVID-19 highlight both the need and the ability to produce high-quality evidence even in the midst of a pandemic. Therapies have not been proven to be effective to this date and current prevention and treatment recommendations are very similar to those suggested in 191822-24.
PREVENTION
Frequently washing hands with soap and water for at least 20 s or using 70% alcohol-based gel solutions. When coughing or sneezing, the use of sneeze etiquette, which consists of covering the nose and mouth with a tissue or the inside angle of the arm. No spitting, and if necessary, use a tissue, put it in a plastic bag, tie it up and throw it away, then wash hands. No face touching with dirty hands, especially the nose, mouth and eyes. Maintain a social distance of at least one meter. Clean and disinfect surfaces and objects of common use in houses, offices, closed spaces, transportation, meeting centers, etc., ventilating and allowing sunlight to enter. Staying at home, according to the health recommendations of each entity. Seek medical attention if any of the symptoms are present (fever over 38°C, headache, sore throat, runny nose, etc.). Avoid contact as much as possible with people who have respiratory diseases. If you need to leave your home, wear a mask that covers your mouth and nose to reduce the risk of infection (Fig. 5).25 As of November 10, 2020, there are 11 vaccine study protocols for COVID-19 in phase III like that of the University of Oxford/Astra Zeneca.26 In December 2020, those of Biontech/Fosun Pharma/Pfizer and Moderna/NIAID were approved by the FDA. Pfizers two-dose regimen vaccine (BNT12622b2) is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS CoV-2 full-length spike protein that conferred 95% protection against COVID-19 in persons 16 years of age or older27.
Convalescent plasma in the management of COVID-19 was not associated with a reduction in progression to severe COVID-19 or all-cause mortality (PLACID Trial)28,29.
PROPOSED PHARMACOLOGICAL TREATMENT
Antimalarial drugs such as hydroxychloroquine and chloroquine have been proposed and showed some benefit in patients with SARS-CoV in 2002, (quinine was used in the 1918 influenza pandemic), protease inhibitor drugs such as lopinavir and ritonavir, RNA polymerase inhibitors such as remdesivir, ribavirin, or favipiravir, interferons such as the ß-1b interferon, interleukin-6 (IL-6) receptor monoclonal antibody such as tocilizumab, interleukin-1(IL-1) receptor monoclonal antibody such as anakinra, drugs that prevent the introduction of SARS-CoV-2 to the host cell such as umifenovir (arbidol), and others such as nitazoxanide that induces the host cells interferon response or an antiparasitic such as ivermectin with broad-spectrum antiviral activity30.
The evidence on the effectiveness of chloroquine and hydroxychloroquine, in addition to being contradictory, is scarce and of low quality. One clinical trial reports that hydroxychloroquine decreases clinical recovery time by 2 days, while another reports no difference in viral clearance between patients receiving and not receiving the anti-malarial.31 The included systematic reviews have contradictory conclusions, but all of them show the low quality of the evidence, one of these studies even published a retraction due to errors in the methodology32.
An important precaution is that the combined use of antimalarials with azithromycin, lopinavir/ritonavir, and remdesivir has been associated with an increased risk of a prolonged QTc interval and arrhythmias. Recently Geleris et al. report an observational study in New York that does not recommend the use of hydroxychloroquine in patients with COVID-19 complicated with ARDS33.
The evidence on antiviral therapy with lopinavir/ ritonavir, oseltamivir, and ganciclovir in patients with severe COVID-19 is weak and contradictory, and its effectiveness in decreasing the risk of progression to ARDS and reducing mortality is unclear. Drugs such as ivermectin and tocilizumab have low quality observational studies that do not allow us to assess the effectiveness and safety in patients with COVID-1934,35.
A recent double-blind controlled study of remdesivir against placebo reports a benefit in patients with COVID-19 in preventing a statistically significant percentage of ARDS complications by administering an initial dose of 200 mg intravenous remdesivir and 100 mg every 24 h over the next 9 days36. However, a subsequent randomized study to evaluate the benefit of remdesivir showed no significant difference between remdesivir and placebo, evaluating results at day 5 and day 10 of treatment37, and the conclusions of the WHO SOLIDARITY study report that remdesivir, hydroxychloroquine, lopinavir, and interferon have little or no effect on hospitalized COVID-1938.
Reyes et al. reported in December 2020 the use of colchicine 0.5 mg orally per day, as a nonsteroidal anti-inflammatory therapy that inhibits E-selectin and L-selectin as well as NLRP3 preventing cytokine storm and platelet aggregation. The use of colchicine, in this randomized, double-blind study against placebo in COVID-19 positive patients, reduced the risk of hospitalization by 25%, mechanical ventilation by 50%, and death by 47%39.
Two observational studies report beneficial effects of glucocorticoid use in patients with a serious COVID-19 disease and a systematic review and meta-analysis on clinical outcomes of the use of corticosteroid in patients with COVID-19 suggesting that its use at low or moderate doses reduces the possibility of mild/moderate to severe disease progression, and mortality40-42.
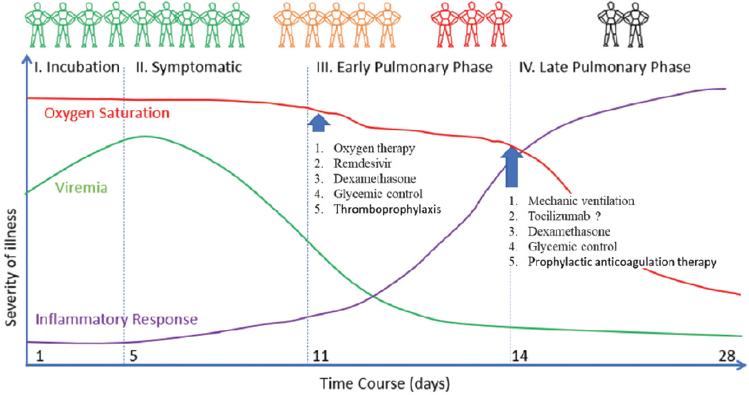
Both the Wuhan University guide and the Surviving Sepsis guidelines recommend oxygen therapy as needed according to hypoxia. It is recommended to start with a nasal cannula and progress to high flow oxygen sources.
The two guidelines included recommend restrictive resuscitation with intravenous fluids (mainly crystalloids), both in ventilated and non-ventilated patients. The use in high volumes may worsen the degree of pulmonary edema, prolong days on the ventilator, ICU stay, and mortality in patients with ARDS. (Fig. 6)43,44.
It is clear that the pathogenesis of COVID-19 involves not only virus replication but also immunomodulation and inflammation. Sequential studies of biomarkers such as interleukin-6, C-reactive protein, ferritin, and D-dimer should help us to better understand the pathogenesis of COVID-19. Combination therapy studies with other antivirals and dexamethasone in appropriate sequence are a high priority, and plans for such studies are already underway.
Sleep associated breathing disorders such as obstructive sleep apnea syndrome (OSAS) and Sleep-Related Hypoventilation Syndromes have not been considered as risk factors in the complication of SARS-CoV-2 infection and that may contribute to the progression from a mild COVID-19 illness to a severe or critical phase with ARDS, including death.
Adult Obstructive Sleep Apnea Syndrome
Diagnostic criteria for OSAS in adults: Criteria A and B must be met45.
-
A) The presence of one or more of the following:
The patient complains of daytime sleepiness, non-restorative sleep, fatigue, or insomnia.
The patient wakes up due to shortness of breath, choking, or suffocation.
Bed partner reports habitual snoring and breathing pauses in the patient during sleep.
The patient has been diagnosed with arterial hypertension, presents mood disorders, cognitive dysfunction, coronary artery disease, ischemic cerebrovascular disease, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus.
-
B) The polysomnography (PSG) record shows (Fig. 7):
Or:

Figure 7 PSG of an obese adult patient, with controlled arterial hypertension, excessive daytime sleepiness, and loud snoring during sleep.
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is characterized by repeated episodes of complete (apnea) or partial (hypopnea) upper airway obstruction that occurs during sleep.
These events result in reduced blood oxygen saturation and usually end in brief, transitory awakenings. By definition, episodes of apnea or hypopnea last a minimum of 10 s. Most events last from 10 to 30 s, but sometimes they persist for a minute or more. These events can occur at any stage of sleep, but most often in stages N1, N2 of non-REM sleep, and R (REM sleep). During REM sleep or when the person is sleeping in the supine position, events are usually longer and associated with a severe decrease in oxygen saturation.
Oxygen saturation usually returns to normal after normal breathing resumes, but may remain low if apnea or hypopnea events are very frequent and prolonged or if there is underlying lung disease. The prevalence of OSAHS has increased to 30% from 1990 to 2010, from 4% in men and 2% in women to 7.5% in men and 4.2% in women. Age is also an important factor. As OSA is most common after age 40 and reaches its peak frequency in individuals over 60 years of age46.
Given that obesity is the main risk factor for the development of OSA, it is expected that as the Mexican population continues to suffer from the severe overweight pandemic considering the BMI (Body Mass Index = mass/height2 = per kg/m2: BMI kg/m2 > 25.00), obesity (BMI kg/m2 > 30.00) and morbid obesity (BMI kg/m² > 40.00), the incidence and prevalence figures of OSA will also increase.
One parameter to consider in addition to BMI is the perimeter of the neck, as the larger the perimeter, the greater the risk of a higher apnea/hypopnea index (AHI) in people with OSAHS. That is, in women with a neck circumference greater than 38 cm and in men with more than 40 cm, the frequency of OSA is higher in these subjects47.
Hypoxia and the changes in sympathetic activity associated with OSA originate: insulin resistance with increased adipokines such as leptin and adiponectin related to pro-inflammatory cytokines such as interleukin-6 (IL-6), the monocyte chemoattractant protein (MCP-1), plasminogen activator inhibitor-1 (PAI-1), or tumor necrosis factor alpha (TNFα), favoring endothelial dysfunction with systemic arterial hypertension, metabolic syndrome, coronary artery disease, or ischemic cerebrovascular disease48-50
Therefore, patients with OSAS have a higher risk of presenting these comorbidities, with an OR for arterial hypertension of 2.89 and for cerebrovascular disease the OR is of 1.58. On the other hand, the most frequent sleep disorder in post-cerebral infarction is OSA with a 62% on the first night51.
The general treatment for OSA is hygienic and dietary measures such as: weight loss, avoiding the use of tobacco, and alcohol or benzodiazepine abuse. Specific treatment is with CPAP (nasal continuous positive airway pressure) which lowers the apnea/hypopnea index (AHI) and prevents chronic nocturnal hypoxemia with decreased superoxide production, and ROS, decreasing endothelial adhesion molecules such as intercellular molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), interleukin-6 (IL-6), and increasing nitric oxide (NO) levels52.
Obesity, OSAS, and Endothelial Dysfunction
Endothelial and metabolic dysfunctions as well as adiposity constitute physiopathological links between an unfavorable lifestyle and the so-called classic and emerging risk factors, among which are arterial hypertension, dyslipidemia, diabetes mellitus, activation of the inflammatory cascade, the prothrombotic state, and a substrate that favors cardiac arrhythmias. Among the subclinical and final consequences the role of overweight and obesity stands out, which reflect visceral adiposity as a central element in the risk and pathogenesis of endothelial dysfunction leading to coronary artery disease, ischemic cerebrovascular disease, and most probably in the complication of COVID-19 favoring a hyperinflammatory reaction to the SARS-CoV-2 response and the immune response with pyroptosis and cytokine storm originating an increase in the exudate at alveolar level with vascular endotheliitis and pulmonary thrombosis (Fig. 8).

Figure 8 Putative causal mechanisms of metabolic and cardiovascular diseases related to obstructive sleep apnea (OSA).
Visceral fat is considered a mere energy deposit with a wide anatomical distribution. In recent years, it has become clear that visceral fat tissue is a true endocrine organ of great activity producing adipokines that intervene in different events that can lead to the development of a metabolic syndrome. Insulin resistance is, for example, a key situation in the progression of the disease and different adipokines induce this resistance directly, such as leptin, resistin, TNFα, and IL-6, by preventing the transduction of the signal produced by insulin, thus inhibiting the transcription and translocation of glucose receptors. The resulting hyperglycemia leads to an increase in the inflammatory process due to the production of reactive oxygen species (ROS)53,54 (Fig. 9).
At the same time, secondary hyperinsulinemia to such resistance causes defects in phagocytic cells by increasing the circulation of bacterial antigens, which have the capacity to activate leukocytes and adipocytes that then release pro-inflammatory cytokines, this being another causal mechanism of inflammation55-57.
Consequences will be increased in patients with COVID-19, if obesity is associated with OSAS or Sleep-Related Hypoventilation Syndromes (Fig. 10). At present, there is no direct evidence to support OSA as an independent risk factor for severe SARS-CoV-2 infection, but some inferences can be made from the data on ARDS. Obesity was shown to be an independent risk factor for developing ARDS among hospitalized patients. In a retrospective study of more than 6,000,000 cases, obstructive sleep apnea has been associated with an increased risk of developing ARDS among patients undergoing surgical procedures. In addition, OSA patients who are hospitalized generally have an increased risk of mortality and morbidity, but the risk is decreased among patients treated with noninvasive ventilation (NIV)58,59.
Conclusions
Hypoxia due to inflammation of the upper airway or lower airway in patients infected by SARS-CoV-2, obesity with or without obstructive sleep apnea (OSA) in the elderly and OSA with dysfunction cerebral glymphatic system during sleep are severe factors that can contribute to the transition from phase I of COVID-19 to Phases II and III with hyperinflammation, acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome, and death. We, therefore, consider the need to carry out prospective clinical studies supported by ambulatory polysomnography and in the shorter term, retrospective studies, to have information based on evidence medicine on the level of risk of OSA in comorbidity and fatality associated with COVID-19.
Hence, we propose to disseminate worldwide the need to question, in patients with initial COVID-19, the history of chronic snoring, the possibility of pauses in breathing during sleep reported by the patients partner and the presence of excessive daytime sleepiness. Particularly in male patients over 60 years of age with obesity and/or diabetes, to have the clinical suspicion of OSA that can be corroborated with an outpatient polysomnography study and establish preventive treatment with colchicine as an anti-inflammatory measure and in case of increased frequency respiratory, dyspnea or O2 saturation less than 89%, indicate the use of non-invasive ventilation, during wakefulness but with greater emphasis during sleep.











 nova página do texto(beta)
nova página do texto(beta)