Introduction
An estimated 50 million people worldwide have dementia, with Alzheimers disease (AD) representing 70-80% of cases.1 It is expected that by the year 2050, 150 million people globally will suffer from some form of dementia1.
In Mexico, the panorama is not very different. We have a rapidly aging population which is transforming the triangular shape of the population pyramid representing the predominance of young people over the elderly, to a rectangular one made up of the same number of young people as seniors; however, the long-term trend is a complete transformation into an inverted pyramid. The prevalence of AD in Mexico is 7.3% and has an incidence of 27.3 (1000 person/year). It is estimated that by the year 2050, the number of Mexicans with AD will be > 3.5 million2.
Dementia is a physical, social, and emotional problem and one of the most important causes of disability and dependency among older adults1. Furthermore, it represents a substantial economic impact. For these reasons, dementia is considered a priority for public health in Mexico and it is also considered a global emergency. Today, more people live with dementia than those who can be cared for by the rest of the population.
At present, AD diagnosis is based on clinical data3. Positron emission tomography scans for Aβ (β-amyloid peptide) and Tau protein are expensive diagnostic tools, only available in some highly specialized centers. Nevertheless, in recent years, three core cerebrospinal fluid biomarkers have been identified: Aβ42 (42-aminoacid form of Aβ), T-tau (total Tau), and P-tau (phosphorylated tau). These biomarkers have reached a specificity and sensitivity ranging between 85 and 90% for the diagnosis of AD and also for the stage of mild cognitive impairment due to AD. Although only through postmortem histopathological findings, it is possible to reach a definitive diagnosis, other emerging biomarkers such as presence of P-Tau in blood, skin, and oral mucosa4-6 are promising tools.
At present, AD treatment is merely symptomatic. Pharmacological treatment involves acetylcholinesterase inhibitors and N-methyl d-aspartate receptor antagonists. They contribute minimally to early stages of disease and they do slow the progression of AD in later stages and provide some symptomatic relief but do not achieve a definite cure7. Considering the high prevalence of the disease, it is necessary to progress in our understanding of its pathogenesis so that in the future, an earlier diagnosis can be achieved and eventually, a progression modifier treatment could be offered to patients.
Current knowledge on the pathogenesis of AD
Based on the histopathological characteristics of the disease, amyloid plaques and neurofibrillary tangles presented by all patients who die from AD, the hypothesis of the amyloid cascade has been formulated as pathogenesis of the disease for almost 30 years8. This theory proposes that the amyloid precursor protein (APP), an integral membrane protein, is abnormally processed to beta-amyloid (Aβ), which forms Aβ plaques that cause neuronal dysfunction and death. Likewise, the toxic concentrations of beta-amyloid favor different conformational changes in the Tau protein, leading to the formation of neurofibrillary tangles, which culminate in structural and functional alterations of neurons (Fig. 1). It has been the most accepted theory, since mutations of several genes such APP and some enzymes that process this protein (presenilins, PSEN1 and PSEN2) are causes of the early-onset familial form, which accounts for 1% of AD cases9. Furthermore, sleep affects Aβ accumulation and clearance through the glymphatic system. For both animal and human models, it has been observed that sleep deprivation causes the augmentation of soluble Aβ10. Nevertheless, a significant limitation of this hypothesis is the fact that the presence of amyloid pathology is not always accompanied by dementia11. In fact, amyloid deposition was present approximately in 25-30% of cognitively intact individuals in their eighth decade12.
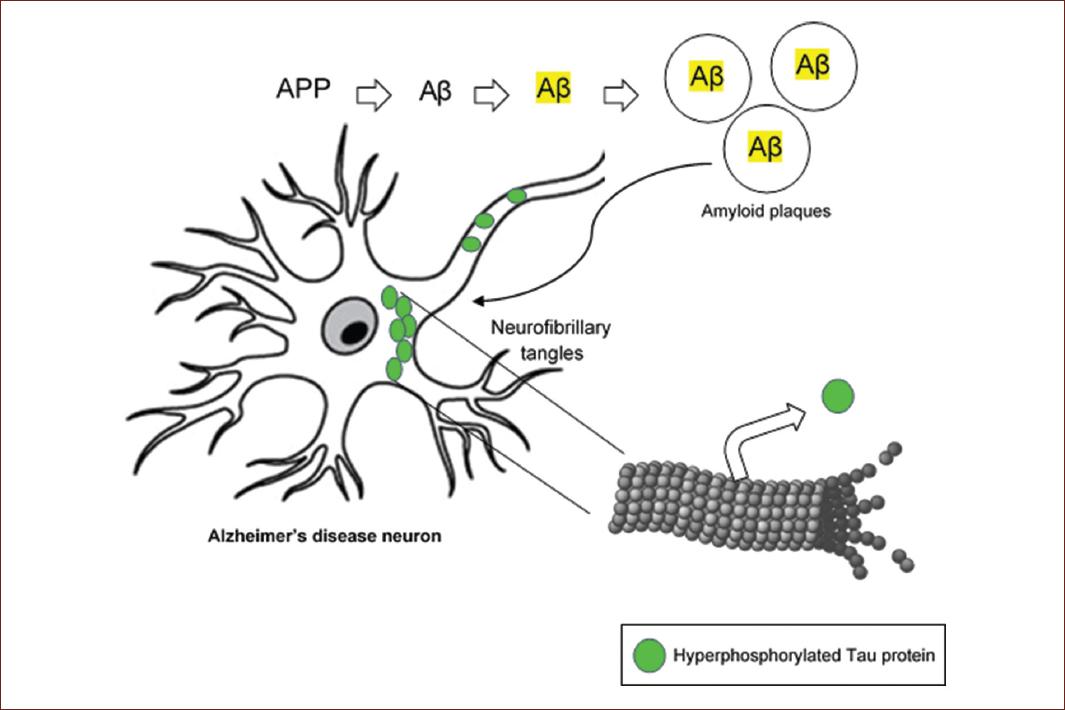
Figure 1 Schematic representation of the amyloid cascade hypothesis. Alzheimers disease would start by the abnormal processing of the amyloid precursor protein to β-amyloid, whose aggregation constitutes amyloid plaques. These abnormal structures would activate Tau protein hyperphosphorylation, its detachment from microtubule and subsequent building neurofibrillary tangles.
On the other hand, the universal finding of hyperphosphorylated Tau protein in neurofibrillary tangles and its high correlation with the degree of cognitive deficit in patients has tilted the balance toward the dysfunction of this protein as a causal disease agent13, However, a group of progressive neurodegenerative diseases called tauopathies that present the pathological conformation of Tau has been documented14. Therefore, despite being part of AD pathognomonic findings, hyperphosphorylated Tau was ruled out as a specific causative AD factor, due to its presence in other neurodegenerative diseases (Fig. 1).
Spread of proteinopathies
Most neurodegenerative diseases, including AD, are characterized by the aggregation of misfolded proteins in different brain regions, which follow a specific pattern for each disease. The presence of this phenomenon, similar to transmissible spongiform encephalopathies, whose causal agent is transforming the conformation of a protein, pointed out to the folding process and functionality of proteins as a cause of neurodegenerative diseases15.
To understand this theory, it would help to remember that the sequence of amino acids conforming the primary structure of proteins contains the folding information into a tertiary structure, which determines their function. The correct protein folding is crucial and several cellular mechanisms avoid abnormal folding. However, under certain post-translational modifications, energy deficit, or alterations of protein degradation pathways, proteins may fold in an alternative and dysfunctional tridimensional shape. It is proposed that neurons assemble these dysfunctional proteins into protein aggregate to focus their energy expenditure in other essential processes; this generates proteostasis alterations with an imbalance between protein synthesis and turnover. Due to the low-energy configuration that misfolded proteins acquire, abnormal folding of the rest of the proteins is favored, which propagates proteinopathy. This process is similar to the behavior of prions and has been demonstrated in the main neurodegenerative diseases16.
New horizons
The different theories proposed about AD pathogenesis continue to leave many unanswered questions about its onset and the evolution of the disease. Although the formation of protein aggregates and their spreading have been widely demonstrated, the factors that trigger alterations of proteostasis leading to the proteinopathy are not fully elucidated yet.
In the late 1990s, neuronal modifications beyond the appearance of neurofibrillary tangles and amyloid plaques were envisaged. Normally, since neurons are terminally differentiated cells, they maintain their quiescent state throughout the whole postnatal life. However, degenerating neurons were surprisingly found to show signs and cell cycle markers of mitosis. It was demonstrated that they emerge from their quiescent state undergoing abnormal reentry into the cell cycle in the direction toward mitosis11. The neurons that initiate AD express cyclin B and nuclear proliferation antigen which allows them to replicate their genome, and thus initiate the cell cycle. The reason for this is an attempt to repair the accumulated DNA damage over the years, which would allow them to survive. However, the complexity of their cytoskeleton and other factors does not allow them to divide, becoming trapped before the mitosis phase (Fig. 2). Neurons that have duplicated their genetic material, becoming hyperdiploid neurons, start appearing in the AD preclinical phase and progressively die throughout the disease17. This theory explains that advanced age is the most decisive risk factor for developing AD. At present, it is well demonstrated that aging per se affects the structure and functionality of nuclear chromatin18, which is related to the accumulation of DNA damage. Aging associated DNA damage is closely related to AD pathogenesis and also to the pathogenesis of cancer19, which has led us to consider that these two diseases, with a high prevalence in senile subjects, could have the same origin: a molecular origin that involves irreversible chromatin changes (Fig. 2).
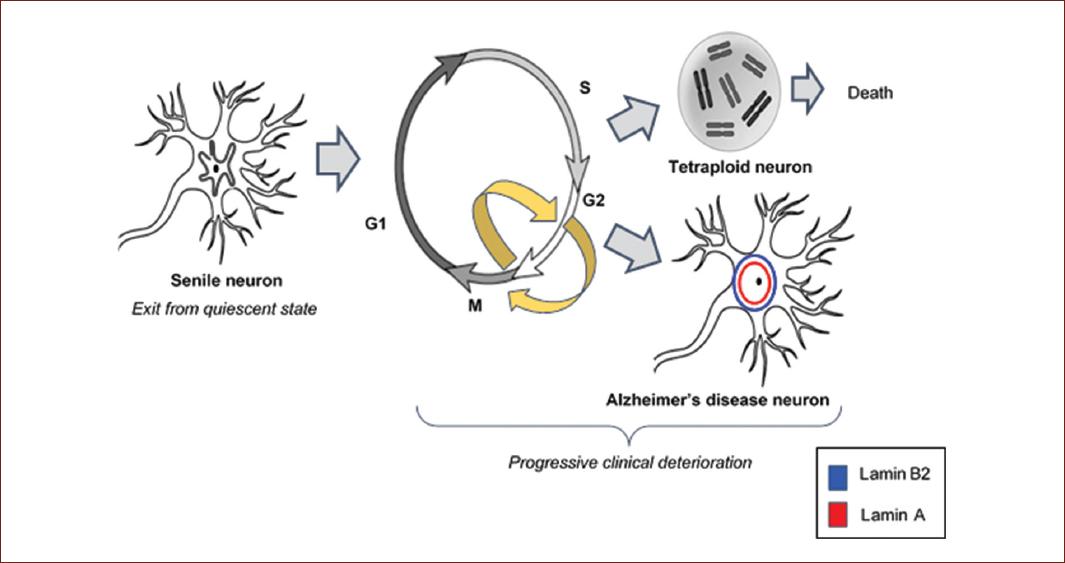
Figure 2 Aberrant cell cycle reentry. Cell cycle reentry by previously quiescent post-mitotic hippocampal neurons is a mechanism to repair DNA damage. Neurons with duplicated genetic material (hyperdiploid neurons), progressively die throughout Alzheimers disease. Neurons that undergo nucleus-skeleton changes survive for years with synaptic dysfunction. These important transformations lead to progressive cognitive deficit.
Tau in the frontier between aging and AD
In light of these antecedents, recent research has analyzed the changes taking place in neurons of the hippocampus during aging and their nuclear transformation in AD. The localization of phosphorylated Tau protein inside the neuronal nuclei is one of the crucial findings that have recently emerged. After being considered a cytosolic protein with an important function by stabilizing the microtubules, its essential role as a nuclear protein was demonstrated20.
First, it was observed that a phosphorylated form of Tau progressively accumulates in the neuronal nuclei during aging21. Second, it was documented that Tau is essential for the healthy aging of post-mitotic neurons of the hippocampus, protecting the genome, and stabilizing the heterochromatin. It achieves this by allowing the adequate compaction of chromatin to silence repetitive DNA sequences and to guarantee the genome regulation22. This ability agrees with its increased presence in neuronal nuclei of senile subjects since, as we mentioned earlier, aging per se results in accumulation of DNA damage.
At early AD stages, Tau gradually disappears from the nucleus, followed by its presence in the neuronal cytoplasm at later stages of the disease, forming neurofibrillary tangles, and it is also found in extracellular aggregates and neuritic plaques. Since Tau protein interacts with the DNA, stabilizing heterochromatin23, the exit of Tau from the nucleus involves chromatin instability and dysregulation of eu- and heterochromatin gene expression24. Furthermore, Tau accumulation impairs RNA translation25 and results in a pathological transformation of the nucleus-skeleton, characteristic of AD, involving global chromatin decondensation26.
Importantly, Tau exit from neuronal nuclei throughout AD culminates in the loss of neuronal plasticity associated to the presence of neurofibrillary tangles. Thus, a series of events that generate the dysfunctional AD neuron involve global decondensed chromatin27, activation of previously silenced gene sequences28, repression of genes characteristic of post-mitotic neurons and the reading of new genes29, alteration of nucleosomal transport,30 and alterations in the translation and transcription of several genes31.
Initial proposals for AD as a laminopathy
Considering that aging itself involves chromatin alterations, much has been speculated about the borderline between healthy aging and AD onset. Over the past decade, based on research work carried out in a Drosophila melanogaster AD model, the nuclear lamin has been found to play an initial and decisive role in AD onset27, even leading to propose that AD would be an acquired laminopathy32. Although both aging and AD are closely related to nuclear lamin dysfunction33, it was still unclear the decisive challenge that leads neurons to an irreversible nuclear transformation that culminates in neurodegeneration and dementia.
The nuclear lamin is a flexible polymer mesh. We could imagine it as a scaffold that contains a diffuse network inside and is surrounded on the periphery by a nuclear lamin. The nuclear lamin is anchored to the cytoskeleton and extends through the nucleoplasm. Its function is to give structure to the nucleus, as well as to regulate chromatin. Its main components are lamin A, lamin B1, lamin B2, and lamin C19. Each of these proteins has independent mechanical properties33. Actually, one important Aβ effect is fragmentation of lamin proteins. In vitro, nuclear lamina deformation and fragmentation are observed in Aβ-treated cells and may contribute to neuronal death34.
It is important to emphasize that neurons, unlike other cells in the human body, do not have lamin A in their nuclear lamin, which gives the nucleus-skeleton greater flexibility35-37. The close and complex relationship of nuclear lamin with chromatin modulates euchromatin and heterochromatin and safeguards the stability of the genome26.
Alterations of the nuclear lamin among young, senile, and AD subjects
Senile neurons possess a more complex nuclear lamin with less flexibility than young neurons. Nevertheless, the absence of lamin A allows them to maintain a certain degree of plasticity in the mechanical communication between their nucleus and cytoskeleton35,36.
Our research group set out to elucidate the pathogenesis of this disease by studying pyramidal neurons of the hippocampus (areas CA1 and CA3) from autopsies of human of healthy subjects of different ages (adults, senile), and AD subjects in different stages of disease (Braak I-VI). Our work demonstrated important changes of the nuclear lamin associated to AD progress that was described in detail in an already published work and outlined below26.
The nuclear lamin from AD hippocampal neurons has been observed to be abnormally complex35,36, but the most surprising finding made by our research group is that AD neurons are the only ones that express lamin A26. This suggests that the presence of lamin A confers greater rigidity to the core. The other nuclear lamin components, lamin B1 and B2, intertwine to construct a scaffold for heterochromatin. Lamin B1 is essential to assemble the different lamins that make up the nuclear lamin26.
Our work demonstrated that lamin B1 is expressed in the nuclear membrane both in young subjects and in AD26. As for lamin B2, it is expressed in healthy, senile, and AD subjects; however, there is an exciting change in its expression. In adult subjects, it has perinuclear expression, but it begins to move to the nucleoplasm in senile subjects, giving rise to two different neuronal populations37. Both the striking findings of the presence of lamin A and the expression changes in lamin B2 raised numerous questions about the impact that nuclear lamin modifications have on the pathogenesis of AD.
Nuclear hypothesis of AD pathogenesis
The comparison of lamins A and B2 expression and of Tau protein in hippocampal neurons of senile and AD subjects suggests a completely new and fascinating theory about the pathogenesis of AD.
Previously, it was known that at early AD stages, two different neuronal populations coexist, one group enters an abortive cell cycle and represents 75-90% of neuronal loss38-40. A second group of neurons does not die but they suffer a transformation of nucleus and cytoskeleton, which triggers hyperphosphorylation and aggregation of Tau.
From our research work, as well as from the great contribution of previous studies, we arrived to the following hypothesis. The expression of lamin B2 in the nucleoplasm indicates that the neurons entered the G1 phase, which coincides with the fact that this expression is observed in healthy senile adults; therefore, we can deduce that the neuron is beginning its effort to repair its DNA, which bears aging associated DNA damage. To survive, post-mitotic neurons generate a change in the nuclear lamin by inducing abnormal expression of lamin A in the inner part of the nuclear lamin and increasing Lamin B2 expression in the nuclear periphery. This restructuration of their nucleus and cytoskeleton increases the rigidity of the nucleus and the degree of viscosity of the chromatin41. The presence of lamin A is the critical difference between senile neurons and AD neurons. In turn, this abnormal expression of lamin A leads to the upregulation of the epigenetic marker H420me3, whose physiological function is to silence perinuclear heterochromatin blocks (Fig. 3).
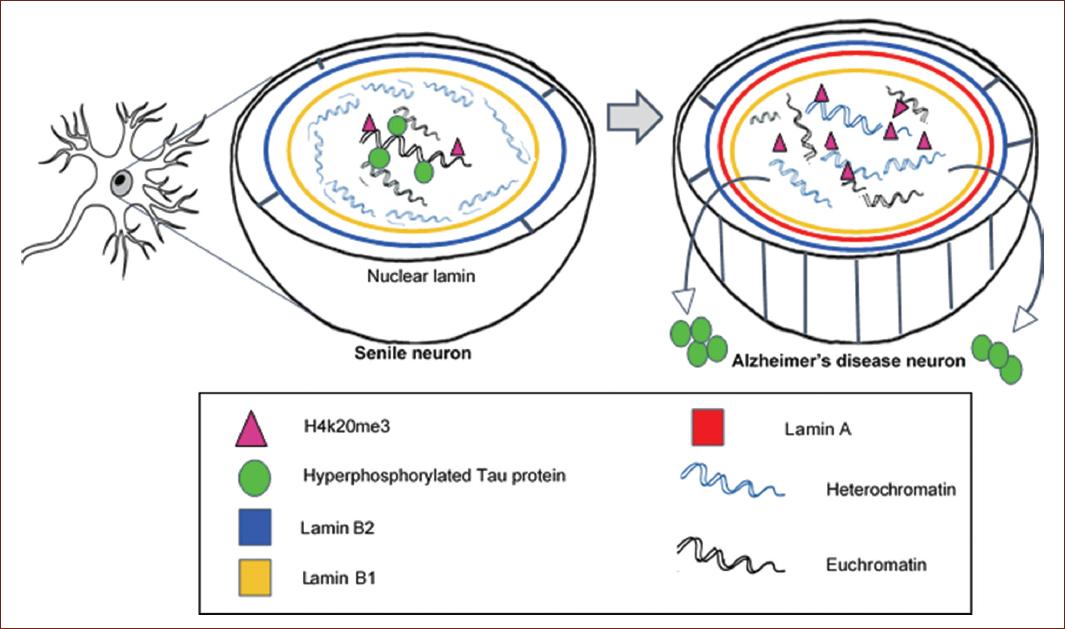
Figure 3 Nuclear hypothesis of Alzheimers disease (AD) pathogenesis. Senile neurons initiate DNA repair by entering the cell cycle, but the complexity of their nucleus-cytoskeleton prevents neurons from dividing and they gradually die. AD neurons survive by triggering an aberrant structural and functional transformation of the nucleus, consisting in lamin A expression and Tau protein transfer to the cytoplasm. Neurofibrillary tangles are consequence of the attempted neuronal repair, and the loss of the protector role of nuclear Tau results in global chromatin disorganization.
Although this tremendous effort to protect the genome is ultimately unsuccessful, since neurons are left cycling in the G2-M phase from where they cannot escape, the loss of neuronal plasticity and synaptic dysfunction is the price that the damaged neuron pays surviving a few years, delaying the inevitable neuronal death26 (Fig. 3).
Perspectives
The above described chain of molecular events provides also an explanation for the lack of success of current AD treatments. Since therapies are targeting molecular AD hallmarks such Aβ plaques and tau hyperphosphorylation, the early nuclear damage and aberrant cell cycle activation underlying the proteinopathy are ignored. In light of this novel pathophysiological mechanism, drugs that could interfere with the cell cycle machinery are candidates for AD management. In this respect, drugs currently used in cancer therapy by targeting cell cycle progression and mitosis are currently tested in animal models of AD42.
Experimental tools against oxidative damage and mitochondrial dysfunction underlying DNA damage are ongoing. However, these treatments are still undergoing clinical trials and many of them have not achieved satisfactory results. It is worth considering that lifestyle and nutritional intervention may be effective primary prevention strategies for AD, since they could reduce the DNA damage accumulation that results in cell cycle activation. Some proposed modifications are healthy nutritive food components, rich in their antioxidant and anti-inflammatory properties; intermittent fasting, which is effective in brain aging by improving metabolic health, and physical activity which reduces mitochondrial dysfunction by activating various transcription factors in bioenergetics processes43.
Conclusion
Cell cycle reentry by post-mitotic hippocampal neurons is a biological mechanism to restore nuclear-cellular homeostasis after accumulation of DNA damage. Aging presents a state of massive DNA damage that does not allow to restore homeostasis. For this reason, post-mitotic neurons make an immense effort to survive or to delay their death, sacrificing important neuronal functions. This survival success is evidenced by the presence of lamin A and the exit of the Tau protein from the nucleus. Nevertheless, AD neurons manage to survive for years, and even decades, with a progressive deterioration of synaptic functionality that leads inevitably to dementia.











 nueva página del texto (beta)
nueva página del texto (beta)


