Introduction
In December 2019, several cases of severe pneumonia caused by a new virus belonging to the family Coronaviridae were reported in Wuhan, the capital city of Hubei Province in China1. On February 11, 2020, the virus was formally named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) on March 11, 2020, declared the novel coronavirus disease (COVID-19) outbreak a global pandemic, infecting more than 34 million people worldwide2. Unfortunately, the global death toll from COVID-19 has recently surpassed 1 million. To date, Mexico has more than 1,280,000 confirmed cases and more than 116,000 deaths3.
Manifestations of SARS-CoV-2 infection include non-specific symptoms such as fever, headache, cough, myalgias, and olfactory disturbances. Some patients progress to severe respiratory failure that may lead to death. Neurological manifestations include cerebrovascular disease, encephalitis, myelitis, acute necrotizing myositis, para-infectious symptoms, and Guillain-Barré syndrome (GBS)4,5.
The first case of GBS associated with SARS-CoV-2 infection was reported in Wuhan in a woman who returned from China that presented acute lower extremity weakness and areflexia that progressed over 3 days to the arms without any systemic symptoms6. Following this case, Italy reported six cases of GBS associated with SARS-CoV-2 infection4,7. Limited information is available regarding Latin American patients with GBS and SARS-CoV-2. Hereby, we present four cases of GBS associated with SARS-CoV-2 infection in Mexico. Patients characteristics are described in table 1.
Table 1 Characteristics of GBS patients associated with SARS-CoV-2 infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 32 | 41 | 40 | 26 |
| Sex | F | F | M | M |
| Comorbidities | None | COPD | Asthma | None |
| Medication | None | None | None | None |
| Neurological symptoms and signs | Progressive and symmetrical weakness of all limbs (72 h) | Progressive and symmetrical weakness of all limbs (96 h), olfactory disturbances (144 h) | Bilateral facial palsy and numbness of both hands (17 days) | Bilateral facial palsy and numbness in both hands (7 days) |
| Respiratory failure | No | No | No | No |
| Clinical GBS variant | Pure motor | Pure motor | Bilateral facial palsy with paresthesias | Bilateral facial palsy with paresthesias |
| Electrophysiological variant | AIDP | Inexcitable | Not performed | Not performed |
| CSF findings | Normal | Normal | Normal | Normal |
| Treatment | IVIG | IVIG | Conservative | Conservative |
| Outcome (Hughes) | Hughes 3 (3 months) | Hughes 1 (3 months) | Hughes 0 | Hughes 0 |
Case 1
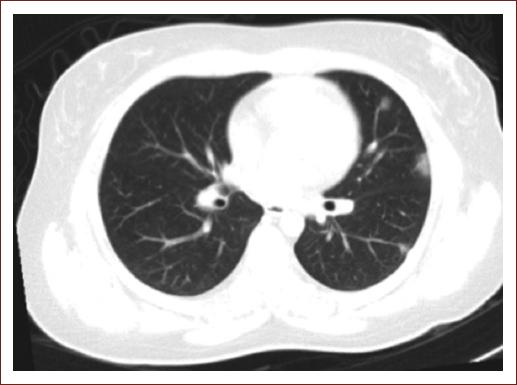
A 32-year-old woman presented to the emergency department with progressive and symmetrical weakness of all limbs that left her wheelchair bound in 48 h. She also had cold sweats, fever, headache, and myalgias 6 days before neurological symptom onset. Neurological examination revealed quadriplegia and generalized areflexia. Admission chest computed tomography found multiple reticular infiltrates (Fig. 1). Laboratory workup was normal, except for elevated lactic acid dehydrogenase (16, 080 IU/L), D-dimer (1313 ng/dL), and creatine phosphokinase (3599 IU/L). Lumbar puncture was normal. SARS-CoV-2 nasopharyngeal reverse transcriptase-polymerase chain reaction (RT-PCR) testing was positive. Nerve conduction studies (NCSs) for motor (ulnar, median, peroneal, and tibial) and sensory nerves (median and sural) were reported as inexcitable. The patient received intravenous immunoglobulin (IVIG) and oxygen supplementation. In follow-up evaluation at 3 months, the patient was able to walk with assistance.
Case 2
A 41-year-old woman with chronic obstructive pulmonary disease history presented to the emergency department with a 4-day history of progressive and symmetrical weakness in all limbs (Fig. 2). Two days before neurological symptom onset, she presented olfactory disturbances, headache, and myalgias. Neurological examination revealed quadriplegia and generalized areflexia. Blood work-up, inflammatory markers, and lumbar puncture were normal. SARS-CoV-2 nasopharyngeal RT-PCR testing was positive. NCS demonstrated demyelinating features (Table 2). The patient received IVIG and oxygen supplementation. At 3-month follow-up evaluation, the patient was able to walk independently.
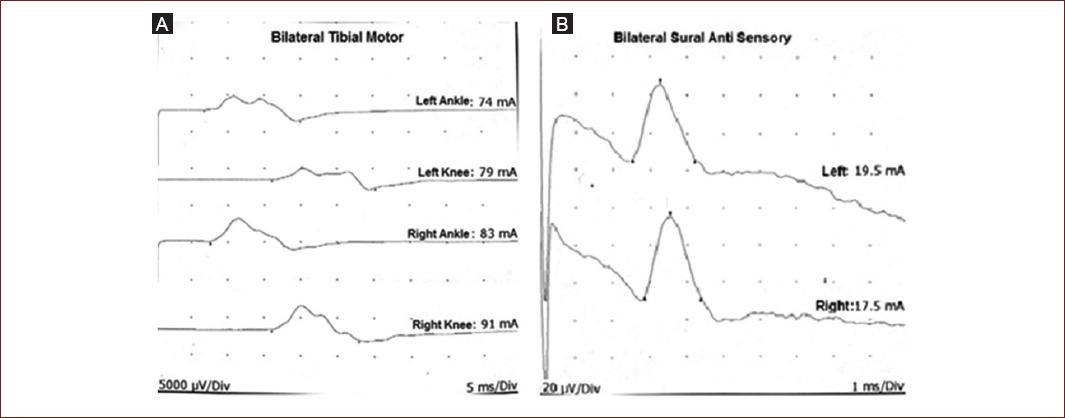
Figure 2 Nerve conduction curves for patient 1. A: Both motor tibial nerves showing increased latency and low compound muscle action potential values. B: Sural nerves with preserved sensory nerve action potentials.
Table 2 Electrophysiological findings in patients 1 and 2
| Motor nerves | Sensory nerves | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Ulnar | Tibial | Peroneal | Median | Sural | |||||||||
| R | L | R | L | R | L | R | L | R | L | R | L | |||
| P1 | Distal latencies | 6.5 | 5.9 | 5.8 | 5.5 | 7.2 | 6.8 | 7.8 | 6.1 | |||||
| Proximal CMAP | 4.2 | 3.8 | 4.5 | 5.4 | 2.5 | 2.1 | 0.8 | 1.3 | SNAP (mV) | 12 | 9 | 12 | 15 | |
| NCV (m/s) | 31 | 32 | 29 | 31 | 33 | 28 | 32 | 30 | NCV (m/s) | 45 | 46 | 43 | 46 | |
| P2 | Distal latencies | NR | NR | NR | NR | NR | NR | NR | NR | SNAP | NR | NR | NR | NR |
| Proximal CMAP | ||||||||||||||
| NCV (m/s) | ||||||||||||||
CMAP: compound muscle action potential; NCV: nerve conduction velocities; SNAP: sensory nerve action potential; NR: not registered; R: right; L: left.
Case 3
A 40-year-old man with asthma history presented to the outpatient clinic with a 3-day history of olfactory and taste disturbances, headache, myalgias, cough, and sore throat. SARS-CoV-2 nasopharyngeal RT-PCR testing was positive. Seventeen days after COVID-related symptoms, he presented bilateral facial weakness and numbness in both hands. Neurological examination revealed bilateral facial palsy with normal strength and absent reflexes. Blood analysis and inflammatory markers were normal. Neither bulbar nerve involvement nor limb motor weakness was present, which led to conservative management with close surveillance. At 1-month follow-up evaluation, the patient persisted only with bilateral facial weakness.
Case 4
A 26-year-old man without relevant medical history presented to the emergency department due to a 7-day history bilateral facial weakness and numbness in both hands. He had a positive nasopharyngeal RT-PCR for SARS-CoV-2 9 days before neurological symptoms initiation. Neurological examination revealed bilateral facial weakness, normal strength, and generalized hyporeflexia. Blood analysis and inflammatory markers were normal. Like the previous case, neither bulbar nerve involvement nor limb motor weakness was present, which led to conservative management with close surveillance. At 1-month follow-up evaluation, the patient persisted only with bilateral facial weakness.
Discussion
GBS is an acute inflammatory polyradiculoneuropathy with a monophasic course. It is characterized by progressive, acute, and symmetrical weakness, sensory findings, occasional cranial nerve involvement, radicular pain, and absent reflexes8. GBS is caused by an abnormal autoimmune response against peripheral nerve gangliosides secondary to molecular mimicry. Up to 70% of the patients have a previous respiratory tract infection in the preceding 1-4 weeks9. GBS outbreaks have been described in association with epidemic and pandemic viral infections, such as influenza A virus subtype H1N1 (A/H1N1), chikungunya virus, Zika virus, Middle East respiratory syndrome-coronavirus, and SARS-CoV10.
To date, no viral SARS-CoV-2 particles have been found in peripheral nerves nor cerebrospinal fluid to establish a strictly direct association between GBS and COVID-19 infection. Ellul et al. defined as probable SARS-CoV-2-associated GBS when: (1) GBS symptoms appear within 6 weeks onset of acute SARS-CoV-2 infection; (2) either SARS-CoV-2 RNA detected in any sample or antibody evidence of acute SARS-CoV-2 infection; and (3) no evidence of other commonly associated causes11.
Most cases have been reported in Asian and European countries, such as China, Spain, and Italy, where SARS-CoV-2 community transmission is highest. The first GBS case associated with SARS-CoV-2 infection reported was a 61-year-old female with generalized weakness with absent reflexes. Afterward, six new cases were reported in Italy. Five patients had acute, progressive weakness, and paresthesia, while one patient had bilateral facial palsy and ataxia4,7.
At present, America has more SARS-CoV-2-positive cases for than other continents. We present four GBS cases related to SARS-CoV-2 infection in different health-care centers in Mexico. Neurological symptoms initiated 2-17 days after the respiratory symptoms. Common clinical findings in our patients were headache, fever, and myalgias. Toscano et al. reported in their series that the latency period between respiratory and neurological symptoms was 5-10 days11.
Cerebrospinal fluid SARS-CoV-2 PCR analysis was not performed in our patients. Nonetheless, in a systematic review that included 30 patients, all cases had a negative CSF report12. Thus, SARS-CoV-2-associated GBS pathophysiology may not be related to direct viral CSF presence in the nerve roots, but by an abnormal autoimmune response to systemic infection10. The age group in or patients ranged from 26 to 41 years, while worldwide reports present cases with patients older than 50 years13.
In our series, two patients presented with progressive, acute, and symmetric weakness and two with bilateral facial palsy. All patients had diminished or absent reflexes. These findings are consistent with clinical findings reported by Toscano7. Literature reports have identified AIDP, AMAN, and AMSAN subtypes in COVID-associated GBS patients. COVID-19 spike protein interacts with the GalNAc residue of GM1 and other gangliosides. After interaction, it anchors to cell surface gangliosides, causing a potential cross-reactivity with peripheral nerve glycolipids. We did not performed anti-gangliosides in our patients10. Patient 1 fulfilled electrophysiological criteria for AIDP variant, while patient 2 was classified as inexcitable. Both patients were treated with IGIV with improvement at 3-month follow-up. Nerve conduction studies were not performed in patients 3 and 4. Furthermore, as both patients presented bilateral facial palsy and no strength compromise, the therapeutic approach was conservative. Patients with GBS diagnosis associated with SARS-CoV-2 infection have been reported to have a good outcome after IGIV or plasma exchange therapy11,13.
Conclusion
SARS-CoV-2 infection is an extremely contagious disease. Fortunately, GBS is likely to be seen increasingly in patients with SARS-CoV-2 infection with few or less severe features of COVID-19. The proportion of patients with GBS is small compared to respiratory manifestations. It is important to consider GBS as a potential manifestation of SARS-CoV-2 infection and recalls that the diagnosis is based mainly on clinical evaluation. Laboratory and CSF analysis, as well as neurophysiologic studies, should be considered as a complement to GBS diagnosis.











 nueva página del texto (beta)
nueva página del texto (beta)