Introduction
Multiple sclerosis (MS) is a chronic degenerative autoimmune disease of the central nervous system characterized by inflammatory demyelination resulting in axonal and neuronal damage. Relapsing-remitting MS (RRMS) being the most common type (85-90%)1,2. Patients with RRMS suffer episodes that can cause fainting, this clinical condition can be disabling3. In Mexico, the prevalence reports ranges from 12 to 30 cases per 100,000 people4.
Various therapies for MS require regular long-term self-injection that can result in patient dissatisfaction, which can severely affect therapeutic adherence and cause a secondary efficacy reduction5. Considering that the worldwide rate of non-adherence for MS is at 44%, which is similar to that of chronic diseases6, oral medications have been introduced to improve adherence and, therefore, have an impact on therapeutic efficiency7. Oral cladribine (2-chloro-2-deoxyadenosine) is an analog of adenosine deaminase resistant to deoxyadenosine8,9. It is a prodrug that requires intracellular phosphorylation, with a chlorine substitution in the purine ring. This protects it from degradation and increases its intracellular time10.
In treatment with cladribine tablets, patients in the 3.5 and 5.25 mg group had fewer magnetic resonance imaging (MRI) lesions11 than those patients in the placebo group, for gadolinium-enhanced T1 lesions (mean 0.11 and 0.12, respectively, vs. 0.91 in placebo) and T2 lesions (mean 0.38 and 0.33, respectively, vs. 1.43 in placebo)12. There is not enough information to directly compare the oral therapeutic strategies available for RRMS in Mexico. The aim of this study was to evaluate the efficacy and safety of cladribine tablets compared to oral therapies currently used in patients with RRMS by means of a systematic review and a network meta-analysis, considering the annualized relapse rate (ARR), T1 lesions, and adverse events that cause discontinuation of treatment.
Methods
Search method
In accordance with the Cochrane methodology, the authors searched for data from 1980 to March 1, 2019, under the criteria of the population, intervention, control, and outcomes question Evaluate the efficacy and safety of cladribine tablets in patients diagnosed with RRMS compared with dimethyl fumarate, fingolimod, and teriflunomide, on PubMed, Cochrane, ScienceDirect, Web of Science, the Health Economic Evaluations Database, EMBASE databases, and regional databases such as LILACs, Scielo Citation Index, Medigraphic, REDALYC, Imbiomed, and Artemisa. The MeSH terms used were MS, RRMS, cladribine, dimethyl fumarate, fingolimod hydrochloride, and teriflunomide, both in English, Spanish, and Portuguese, limited to controlled clinical that included oral disease-modifying therapies.
Inclusion and exclusion criteria
Primary data sources were articles from randomized controlled clinical trials (RCTs). To avoid bias, study selection and data extraction were performed by two independent reviewers. RCTs assessing the effect of cladribine tablets and dimethyl fumarate, fingolimod, or teriflunomide in direct comparison with placebo for the treatment of MS or RRMS were included, and a third reviewer provided consensus when there was disagreement on the inclusion of an article.
Data extraction information
Information was recorded on study design, selection criteria, population, patient characteristics, ARR, T1 lesions, and adverse events.
Quality assessment
The process of rating the quality of the best available evidence in the clinical studies was assessed following the approach proposed by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Working Group and in accordance with the GRADE Handbook.
Outcomes
Efficacy evaluation was performed based on the decrease of ARR and the change in the mean number of gadolinium-enhanced T1 lesions in the MRI. The safety profile was assessed by the number of patients who discontinued treatment due to adverse events.
Statistical analysis
Indirect comparisons were calculated using a network meta-analysis, since this is the most appropriate way of summarizing data to provide a series of unbiased effects obtained from direct and indirect comparisons. A random effects model was used as this is more appropriate than fixed effect models when there is heterogeneity between patient populations and between trials. To ensure a closed network, a placebo was used as a common point. Three interventions were used as comparators: dimethyl fumarate, fingolimod, and teriflunomide versus cladribine tablets; each study had both an intervention and a placebo. Direct evidence of the defined outcomes of each study was incorporated. Statistical significance was determined as p > 0.05. All calculations were performed with the software R version 3.5.2.
Main results
Search results
A total of 1034 articles were identified in the systematic review of the included databases. After duplicated removal, 761 papers were considered. Twenty-six of the potentially relevant articles were assessed for eligibility, and finally, seven clinical trials that met the efficacy and safety criteria were included (Chart 1). The characteristics of included studies are summarized in table 1.
Table 1 Included articles characteristics
| Study | Study design | Participants | Intervention and dosing | n | Duration (Months) | Reported outcomes |
|---|---|---|---|---|---|---|
| CLARITY16 NCT00213135 | Multicentric RCT Phase III |
Adults. RRMS McDonald criteria, EDSS (0-5.5). At least one relapse in the past 12 months. | Placebo Cladribine 3.5 mg Cladribine 5.25 mg |
437 433 456 |
22 | ARR, FRR. Time to first relapse. Mean number of gadolinium-enhanced T1 lesions, weighted active lesions on T2 and combined single lesions. Incidence of treatment emergent adverse events. |
| FREEDOMS15
NCT00289978 |
Double-blind randomized, placebo-CT, Phase 3 | Adults. RRMS McDonald criteria, EDSS (0-5.5). | Placebo Fingolimod 0.5 mg Fingolimod 1.25 mg |
418 425 429 |
24 | ARR. Time of disability progression. Number of gadolinium-enhanced lesions. |
| FREEDOMS II17
NCT00355134 |
Double-blind randomized, placebo-CT, parallel groups, multicentric Phase 3. | Adults. RRMS McDonald criteria, EDSS (0-5.5). | Placebo Fingolimod 0.5 mg Fingolimod 1.25 mg |
355 358 370 |
22 | ARR. Change percentage in brain volume.
Time of disability progression. Number and volume of gadolinium-enhanced T1 lesions. Adverse events. |
| TEMSO18
NCT00134563 |
Double-blind randomized, placebo-CT, parallel group, Phase 3 | Adults. RRMS McDonald criteria, EDSS (0-5.5). At least two relapses in the previous 2 years or one relapse in the previous year, but not within 60 days before randomization. | Placebo Teriflunomide 7 mg Teriflunomide 14 mg |
363 365 358 |
25 | ARR. Disability progression. Total volume of the lesion. Number of unique active lesions. Adverse events. |
| TOWER19
NCT00751881 |
Double-blind randomized, placebo-CT, Phase 3 | Adults. RRMS McDonald criteria, EDSS (0-5.5). At least one relapse in the last year or two relapses in the last 2 years and none in the 30 days prior to randomization. | Placebo Teriflunomide 7 mg Teriflunomide 14 mg |
389 407 372 |
11 | ARR. Time up to 12 weeks of sustained accumulation
of disability. Adverse events. |
| DEFINE20
NCT00420212 |
Double-blind randomized, placebo-CT, Phase 3 | Adults. RRMS McDonald criteria, EDSS (0-5.5). At least one relapse the year before randomization. | Placebo Twice daily BG-12 240 mg Three times a day BG-12 240 mg |
408 410 416 |
24 | Relapses. Number of gadolinium-enhanced lesions. Time of disability progression. Adverse events. |
| CONFIRM21
NCT00451451 |
Double-blind randomized, placebo-CT, Phase 3 | Adults. RRMS McDonald criteria, EDSS (0-5). At least one relapse in the past 12 months or at least one gadolinium-enhanced lesion 0-6 weeks before randomization. | Placebo Twice daily BG-12 240 mg Three times a day BG-12 240 mg Glatiramer acetate 20 mg |
363 359 345 350 |
22 | ARR. Number of new T2 hypertensive lesions or
increasing number of T2 lesions, T1-enhanced
images. Adverse events. |
RCT: randomized controlled clinical trial; EDSS: expanded disability status scale; ARR: annualized relapse rate; FRR: free relapse rate.
Table 2 shows population data by intervention and cladribine dosage groups included in the analysis: cladribine tablets 3.5 mg, dimethyl fumarate 240 mg twice daily, teriflunomide 14 mg, and fingolimod 0.5 mg. The posology of interventions was validated through the Basic Table and Catalogue of Health Sector Inputs (CBCISS) of the General Health Council (CSG) for the Mexican population.
Table 2 Population characteristics
| Reference | Clinical form of the disease | Study arms | Sample size | Age | % women |
|---|---|---|---|---|---|
| Giovannoni et al., 201016 | RRMS | Placebo | 437 | 38.7 ± 9.9 | 288 (65.9) |
| Cladribine 3.5 mg | 433 | 37.9 ± 10.3 | 298 (68.8) | ||
| Cladribine 5.25 mg | 456 | 39.1 ± 9.9 | 312 (68.4) | ||
| Kappos et al., 201015 | RRMS | Placebo | 418 | 37.2 ± 8.6 | 298 (71.3) |
| Fingolimod 0.5 mg | 425 | 36.6 ± 8.8 | 296 (69.6) | ||
| Fingolimod 1.25 mg | 429 | 37.4 ± 8.9 | 295 (68.8) | ||
| Calabresi et al., 201417 | RRMS | Placebo. | 355 | 40.1 ±8.4 | 288 (81) |
| Fingolimod 0.5 mg | 358 | 40.6 ± 8.4 | 275 (77) | ||
| Fingolimod 1.25 mg | 370 | 40.9 ± 8.9 | 281 (76) | ||
| O´Connor et al., 201118 | RRMS | Placebo. | 363 | 38.4 ± 9.0 | 275 (75.8) |
| Teriflunomide 7 mg | 365 | 37.4 ± 9.0 | 255 (69.7) | ||
| Teriflunomide 14 mg | 358 | 37.8 ± 8.2 | 255 (71.0) | ||
| Confavreux et al., 201419 | RRMS | Placebo | 389 | 38.1 ± 9.1 | 273 (70) |
| Teriflunomide 7 mg | 407 | 37.4 ± 9.4 | 300 (74) | ||
| Teriflunomide 14 mg | 372 | 38.2 ± 9.4 | 258 (69) | ||
| Gold et al., 201220 | RRMS | Placebo | 408 | 38.5 ± 9.1 | 306 (75) |
| Twice a day BG-12 240 mg | 410 | 38.1± 9.1 | 296 (72) | ||
| Three times a day BG-12 240 mg | 416 | 38.8 ± 8.8 | 306 (74) | ||
| Fox et al., 201221 | RRMS | Placebo | 363 | 36.9 ± 9.2 | 251 (69) |
| Twice a day BG-12 240 mg | 359 | 37.8 ± 9.4 | 245 (68) | ||
| Three times a day BG-12 240 mg | 345 | 37.8 ± 9.4 | 250 (72) | ||
| Glatiramer acetate 20 mg | 350 | 36.7 ± 9.1 | 247 (71) |
RRMS: relapsing-remitting multiple sclerosis.
From the seven selected studies, data from the annual relapse rate, the average of gadolinium-enhanced T1 lesions, were extracted when available (gadolinium-enhanced T1 lesions data were not available for the TOWER study); for safety data, adverse events that led to the interruption of the study drug were evaluated; this was presented as a rate (Table 3).
Table 3 Data included in the meta-analysis
| Annualized relapse rate | Gadolinium-enhanced T1 Lesions | Adverse events leading to discontinuation of the study drug | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Intervention | Placebo | Intervention | Placebo | Intervention | |||||||
| Study | n | Rate | n | Rate | m- | SD | µ- | SD | n | Rate | n | Rate |
| CLARITY16 | 437 | 33% | 433 | 14% | 0.91 | 2.10 | 0.12 | 2.7 | 435 | 2.07% | 430 | 3.49% |
| FREEDOMS15 | 418 | 40% | 425 | 18% | 1.1 | 2.40 | 0.2 | 0.80 | 418 | 7.66% | 425 | 7.53% |
| FREEDOMS II17 | 355 | 40% | 358 | 21% | 1.2 | 2.97 | 0.4 | 1.84 | 355 | 10.42% | 358 | 18.44% |
| TEMSO18 | 363 | 54% | 358 | 37% | 1.33 | 2.96 | 0.26 | 1.16 | 360 | 8.06% | 358 | 10.89% |
| TOWER19 | 388 | 50% | 370 | 32% | - | - | - | - | 385 | 6.23% | 371 | 15.63% |
| DEFINE20 | 408 | 36% | 410 | 17% | 1.8 | 4.20 | 0.1 | 0.60 | 408 | 13.48% | 410 | 15.85% |
| CONFIRM21 | 363 | 40% | 359 | 22% | 2 | 5.60 | 0.5 | 1.70 | 363 | 10.47% | 359 | 12.26% |
μ- average; SD: standard deviation.
Patient characteristics
Studies were conducted from 2010 to 2014 with similar demographic characteristics, all studies included patients diagnosed with RRMS; as for study design, treatment arms of all studied had the common point a placebo group. All studies included a high percentage (65.9-81%) of female patients (Table 2).
Outcomes report
Comparisons of cladribine tablets with dimethyl fumarate, fingolimod, and teriflunomide were made with efficacy, on the decrease of ARR and the change in the mean number of gadolinium reinforced T1 lesions in the MRI, and safety criteria data extracted through the systematic review.
ARR
Cladribine tablets showed no statistically significant differences with regard to the decrease of ARR compared to dimethyl fumarate and fingolimod, however, a lower relapse rate is shown with cladribine tablets when compared to placebo and teriflunomide (Chart 2).
Gadolinium-enhanced T1 lesions
In relation to the mean number of gadolinium-enhanced T1 lesions, treatment with cladribine reported a lower number of lesions when compared against dimethyl fumarate or placebo (Chart 3). This difference was statistically significant. On the other hand, no statistically significant differences were identified when comparing treatment with cladribine with fingolimod (−0.08 [−0.35; 0.19]) and teriflunomide (−0.28 [−0.64; 0.08]).
Adverse events that lead to a discontinuation of study drugs
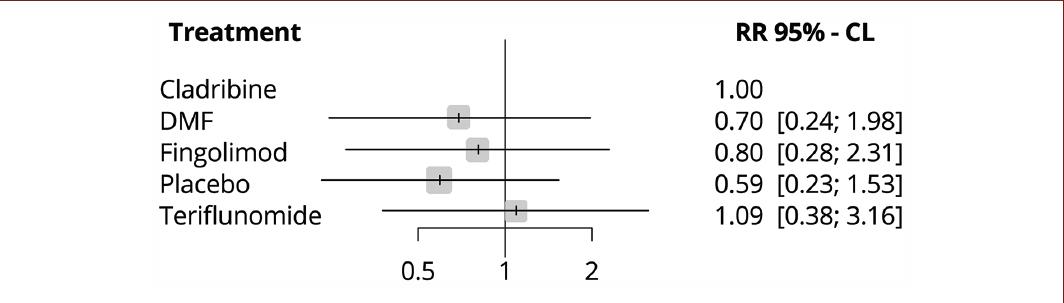
No significant differences were found between cladribine tablets and the other evaluated treatments (Chart 4). In this case, the null effect is represented by the number one.
Discussion
In the absence of randomized clinical studies comparing all interventions for RRMS, a network meta-analysis is a plausible alternative for obtaining relative efficacy estimators. In Mexico, there are very few studies evaluating the efficacy and safety of treatments for MS.
A network meta-analysis by Siddiqui et al. (2018) in patients with RRMS showed that oral cladribine is among the most effective disease-modifying treatments and has an adequate safety profile comparable to other treatments, it also presents a significant reduction in relapse rate compared to teriflunomide and even parenteral drugs13. In addition to this, Papadopoulos et al. conducted a safety analysis on the likelihood to help or harm, defined as the ratio of number needed to harm to the number needed to treat with respect to adverse events causing discontinuation of treatment (NNTH AE-D), which showed favorable evidence for cladribine (72 [95% CI 27.9 to −129.5])14. In this context, our findings are consistent with published reports of cladribine and its safety profile.
In relation to the ARR, cladribine tablets had no significant difference in its effect on relapses compared with the other interventions, however, it had a statistically significant when compared to teriflunomide. The CLARITY study reports an effect size with a greater than 50% decrease in annual relapses, a decrease in disability of up to 30%, and the effect was consistent in sub-population analysis.
The analysis for the gadolinium-enhanced T1 lesions outcome found that the effect of cladribine was comparable to those presented with fingolimod15. Although there is no significant difference between cladribine, fingolimod, and teriflunomide, it must be taken into to consideration that the reported ARR is a stronger measure of efficacy compared to the number of T1 lesions.
The evidence provided by the therapeutic options individually, allows us to put the agents that are used on a daily basis into context, seen in a broader way. This review of oral administered drugs makes it possible to assess important clinical outcomes, while at the same time taking into account that the difference between the characteristics of each drug may affect the clinical outcome. At present, a range of disease-modifying drugs with different mechanisms of action is available, with simplified dosages and periodicity schedules. Cladribine tablets are a therapeutic option that offers the expected therapeutic effect, with an annualized administration scheme that confers comfort to the patient and his caregiver, which undoubtedly favors therapeutic adherence. Cladribine tablets reach quickly and steadily its effect on lymphocytes after an administration, resulting in a good efficacy, safety, and proven tolerability profile.
The specific evidence establishes that all interventions require careful patient selection. According to the safety profile and tolerability of cladribine tablets, in the CLARITY study16 due to its dose-dependent mechanism action, the most common adverse effect was lymphopenia, increasing the risk of an opportunistic infection; however, there are no reports of progressive multifocal leukoencephalopathy (PML), bradycardia, or macular edema attributable to cladribine tablets on patients with MS (Table 4). In relation to the safety profile, this meta-analysis shows no statistically significant differences in adverse effects, but simply a different pattern.
Table 4 Safety profile of oral drugs
| Drug | Short-term side effects | Long-term efficacy | Long-term side effects | Important safety aspects |
|---|---|---|---|---|
| Fingolimod | Bradycardia, average of 8 bpm during the first infusion (2.3%). | Data to 7 years: 84-96% free of gadolinium lesions, 70% free of T2-weighted lesions. | No new aspects to known side effects of crucial tests. | Herpes zoster infection in a small number of patients. |
| Macular edema. | PML risk 1/18.000. | |||
| Elevation of liver function enzymes. | Average PBVC: −2.8 for more than 84 months. | |||
| Mild infections. | ||||
| Herpes zoster infection. | ||||
| Dimethyl fumarate | Flushing or redness. Gastrointestinal irritation. Lymphopenia. |
ARR from years 1-5: 0.202, 0.163, 0.139, 0.143, and 0.138. | PML, so far 5 patients > 230,000 who have been treated with DMF, some cases reported with FUMADERM. | PML risk of 1/50,000. In people > 50 years, early lymphocyte reduction is associated with an increased risk of PML. |
| Teriflunomide | Asymptomatic increase of alanine aminotransferase. | 9 years of TEMSO follow-up. | No pattern of malignancies, especially hematologic cancers such as leukemia or lymphoproliferative tumors. | |
| Headache. | 55% relapse free. | |||
| Diarrhea. | Stable EDSS scale average > 50% without progression. | |||
| Hair thinning. | ||||
| Nausea. | ||||
| Cladribine | Lymphopenia. | Data not available | Data not available | No reported cases of PML in multiple sclerosis. |
| Herpes zoster infection (< 10%). | ||||
| There is no increased risk of malignant tumors. |
*PML: progressive multifocal leukoencephalopathy (adapted from Faissner and Gold, 2018)22.
Limitations and strengths of the study
The main limitation of the study remains that on Mexican population, regarding this disease, information is poor or scarce; so the results of the analysis must be interpreted with caution. Nevertheless, international literature did not provide randomized clinical trials that would allow direct comparisons, making an indirect comparison an alternative to explore the existent and limited alternatives.
The main strength of the study is the use of a network meta-analysis with a random effects model that allows homogenizing the main biases within the analysis to make indirect comparisons. The selection of the articles was carried out by specialists on the subject and in the event of any lack of concession, a third reviewer intervened.
Conclusion
Cladribine tablets demonstrated efficacy in terms of decrease of ARR and gadolinium-enhanced T1 lesions made in contrast with patients with long-term RRMS, as well as a good safety profile and tolerability that promote therapeutic adherence, becoming an appropriate therapeutic option for patients with RRMS. It is important to evaluate the different therapeutic interventions from a standardized perspective for an appropriate treatment selection that positively delays or modifies the natural course of this disease and can contribute to the quality of life of patients with RRMS.
Funding
The preparation of this review was supported by Merck external funding. This article was made with the full autonomy of the authors LRM, GVS, DAO, BLYO, and SMH.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.











 nova página do texto(beta)
nova página do texto(beta)