Introduction
Cervical dystonia (CD) is the most frequent form of focal dystonia found in neurological practice, despite its prevalence is relatively low (ranges from 5/100,000 to 57-280 per million)1,2, and is characterized by involuntary and uncontrollable contractions of specific muscles, leading to an abnormal placement of the head, neck, and shoulders, which could be painful2,3. Abnormal neck postures in CD could take four major forms: head rotation (torticollis), head tilt (laterocollis), head bent forward (anterocollis), and head bent backward (retrocollis)4.
Botulinum neurotoxins (BoNTs) are considered the most widely used therapeutic agents for movement disorders, pain disorders, and autonomic dysfuntion4; injections of BoNT type A (BoNT-A) are first-line treatment and although their efficacy to maintain and control of symptoms has been assessed5 in RCTs, there is little evidence of the effectiveness of long-term BoNT-A in patients with CD in real life. Thus, the aim of this paper was to evaluate satisfaction and clinical response in patients from Mexico and Brazil with CD in clinical practice.
Methods
This is a sub-analysis of data obtained from INTEREST IN CD2 study (NCT01753349), a prospective, international, 3-year longitudinal, observational study aiming to document long-term satisfaction, and the course of idiopathic CD patients treated with repeated BoNT-A, conducted during 2012-2017 across 38 countries, whose methodology and baseline results are specified in the previous publications3,6. As differences in clinical characteristics and practice according to the geographical region have been described at baseline7, this report includes only data from the follow-up of patients in Latin America, specifically Mexico and Brazil. In brief, the main inclusion criteria required patients with legal age of each country and with diagnosis of idiopathic CD were enrolled. Subjects with a prior prescription of treatment with any formulation of BoNT-A in routine clinical practice were considered. The prescription - new or repeated - was made before and regardless of the possibility of being included in the study. Furthermore, to avoid any bias due to prior treatment, the last application should have been at least 12 weeks before to the admission to the study, centers included eight consecutive patients during consultations during a time of period or according to a predefined frequency if consecutive were not possible.
Clinical assessments were made at the beginning of the study and also at each injection visits, before the application of BoNT-A was made. An electronic case report form was used for collecting data regarding sociodemographic characteristics, medical history, as well as details of BoNT-A administration (number of muscles, dose, injected volume, and number of applications).
Of the main outcomes, it was considered patient's satisfaction with symptom control, considered as today's satisfaction at the time of each visit and which was evaluated with a 5-point Likert scale; possible answers included completely satisfied; rather satisfied; neither satisfied nor dissatisfied; rather dissatisfied; and completely dissatisfied. Moreover, clinical outcomes included functionality, which was assessed using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS)8.
The study was conducted in compliance with the Guidelines for Good Pharmacoepidemiology Practices, the International Society for Pharmacoepidemiology and also in accordance with ethical international guidelines and approvals in this context, ethical approval was ensured for each participating country.
The statistical analyses reported in this manuscript are primarily descriptive, absolute, and relative frequencies expressed as number and percentage and their 95% confidence interval are presented for qualitative variables; meanwhile, mean and standard deviation and range in some cases were used to summarize quantitative data. Furthermore, mean differences were calculated and reported.
Results
A total of 82 patients with CD were enrolled in 10 sites distributed in Latin America; three patients were excluded because they reported no post-baseline visit; thus; 79 subjects were considered for this analysis; from Mexico (n = 40) and Brazil (n = 39), of which 93.7% (n = 74) were diagnosed as sporadic; 6.3% (n = 5) familial. About 64.6% and (n = 51) were female. From all, 26.9% reported being unemployed of whom; 33.3% due to a CD or its associated symptoms. Other sociodemographic and treatment characteristics are described in table 1.
Table 1 Demographics and baseline characteristics
| Variable | Total sample (n = 79) | Mean CI (95%) |
|---|---|---|
| Age, years | 56.2 ± 17.6 | (53.2-59.3) |
| Body weight, kg | 67.7 ± 12.1 | (64.9-70.4) |
| BMI, kg/m2 | 26.1 ± 4.7 | (25.0-27.2) |
| Time since diagnosis, years | 9.7 ± 9.9 | (7.5-11.9) |
| Time since first injection, months | 79.0 ± 60.2 | (63.2-94.8) |
| Time since last injection, months | 7.3 ± 9.0 | (5.1-9.4) |
| Number of injection cycles since BoNT initiation, cycles | 9.2 ± 4.9 | (7.7-10.8) |
| Predominant head/neck pattern, % (n) | ||
| Rotation | 51.9 (41) | (41.1-62.6) |
| Laterocollis | 27.8 (22) | (19.1-38.6) |
| Retrocollis | 15.2 (12) | (8.8-24.9) |
| Anterocollis | 3.8 (3) | (0.8-11.0) |
| Lateral shift of the column | 1.3 (1) | (0.07.5) |
| Dose, units | ||
| AboBoNT-A (n = 67) | 599.7 ± 238.1 | (541.7-657.8) |
| IncoBoNT-A (n = 2) | 300 (NA) | - |
| OnaBoNT-A (n = 1) | 175.8 (NA) | - |
| Number of injection cycles during study | 9.01 ± 3.0 | (7.3-8.7) |
| Duration of injection intervals, days | 118.4 ± 24.5 | (112.9, 123.8) |
| Number of injected muscles | 7.45 ± 4.2 | (6.4-8.5) |
Data are reported as mean ± standard deviation or as percentage (n) if quantitative or qualitative variable.
aboBoNT-A: abobotulinumtoxin A; incoBoNT-A: incobotulinumtoxin. A; onaBoNT-A: onabotulinumtoxin A, CI: confidence interval.
Most subjects were previously treated with BoNT-A (88.6%; n = 70). Furthermore, 11.4% (n = 9) of patients were taken concomitant medications before BoNT-A injection and 29.1% (n = 23) started it with the treatment to control pain related to CD (43.5%; n = 10), muscle spasms (30.4%; n = 7), and for other symptoms (47.8%; n = 11).
With respect to study treatment, the mean number of applications of BoNT-A was 7.9 ± 3.01 in an average of 7.5 ± 4.3 injected muscles, of which the most common at any visit was splenius capitis (82.7-97.1%) and sternocleidomastoid (77.1-84.8%), followed by the trapezius (62.5-72.3%), levator scapulae (28.6-61.1%), and scalene group (44.3-57.7%), reported with its minimum and maximum range. Overall, 84.8% (n = 67) received abobotulinumtoxin A; 2.5% (n = 2) and 1.3% (n = 1) onabotulinumtoxin A and incobotulinumtoxin A, respectively, and the rest 11.4% got a different BoNT-A at baseline or follow-up.
Regarding today's satisfaction rates evaluated at the time of each of the follow-up visits, through time are presented in figure 1 where it highlights an increase of 88% in the proportion of subjects completely satisfied. At baseline, 55.7% were satisfied (considering Likert score as completely satisfied/rather satisfied), which increased to 86.1, 84.1, 83.3, and 86.2% at 6, 12, 18, and 24 months, meanwhile at month 36 were 90.2% and at the last assessment at 36 month, the proportion was of 87.0%, which implies an improvement of 56.2% during the 3-year follow-up.
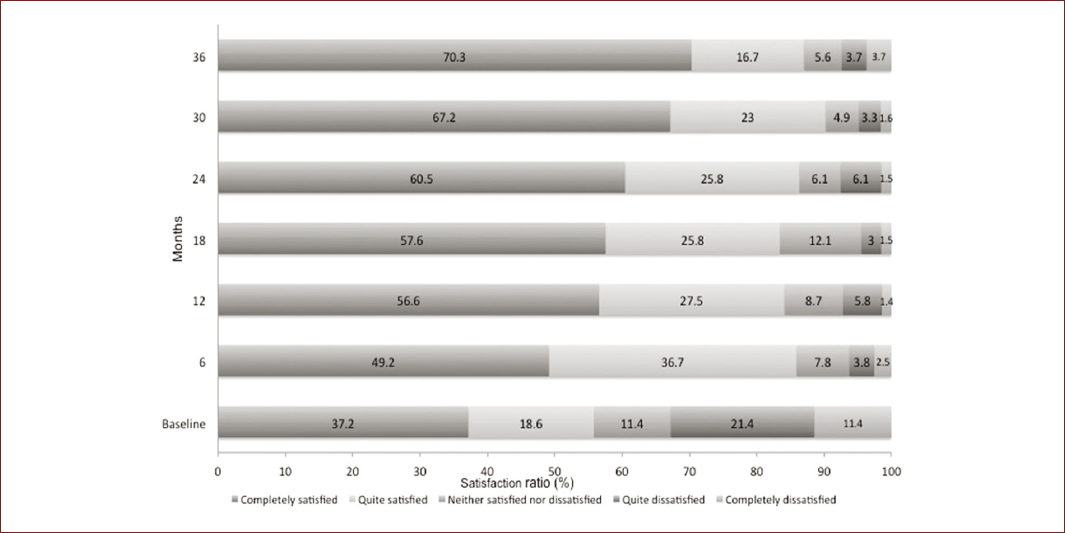
Figure 1 Degree of today's satisfaction by time period of patients with CD treated with BoNT-A during 36 months of follow-up*.*Considering different number or patients in each time; Baseline figures include patients with a previous treatment with BoNT (n = 70); at 6 months (n = 79); at 12 months (n = 69); at 18 months (n = 66); at 24 months (n = 66); at 30 months (n = 61); at 36 months (n = 54).
Mean TWSTRS scores appeared to continually decrease over the course of the study (from 40.2 at baseline to 23.2 at 3 years), as seen in table 2, representing a final difference of −15.4 points from baseline to the end of the follow-up (Fig. 2). When considering a TWSTRS severity score ≥ 15 points, 79.7% were classified as severe at baseline. At 6 months, there was a decrease in that figure to 64.6%; which remained in 59.4, 65.2, 54.1, and 59.1% at 12, 18, 24, and 30 months, respectively; with 48.1% of patients classified as severe at 36 months, suggesting an improvement for 31.6% who initially had a TWSTRS score ≥ 15 points.
Table 2 Mean score for TWSTRS by time period
| Time period during follow-up | Score TWSTRS | Mean CI (95%) |
|---|---|---|
| Baseline | 40.2 (14.1) | (37.01-43.34) |
| 6 months | 33.1 (13.5) | (30.1-36.11) |
| 12 months | 30.9 (13.0) | (27.8-34.01) |
| 18 months | 29.2 (12.5) | (26.2-32.4) |
| 24 months | 27.6 (12.3) | (24.6-30.6) |
| 30 months | 26.6 (12.6) | (23.4-29.8) |
| 36 months | 23.2 (11.5) | (20.1-26.4) |
Data are presented as mean (standard deviation).
CI: confidence interval; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale.
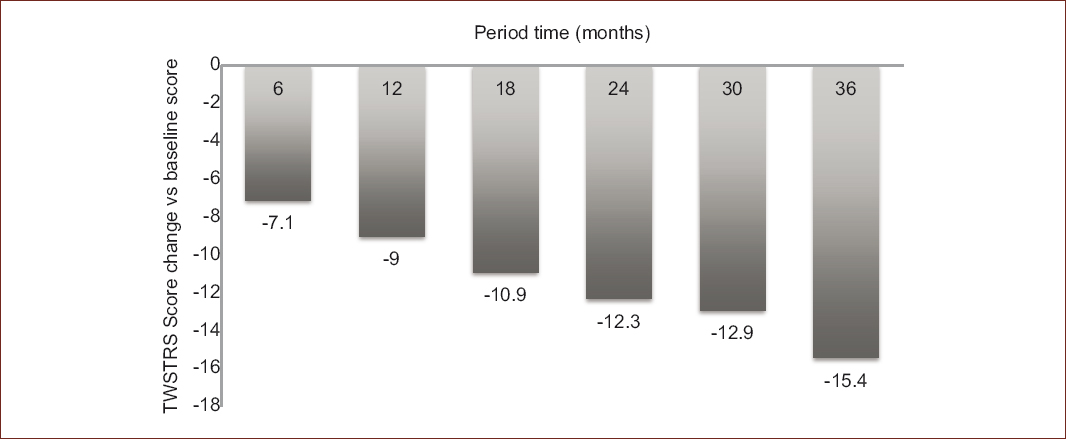
Figure 2 Changes in the TWSTRS total score by time period among patients with CD treated with BoNT-A*.*Considering different number or patients in each reported change, versus baseline (n = 79); at 6 months (n = 79); at 12 months (n = 69); at 18 months (n = 66); at 24 months (n = 66); at 30 months (n = 61); at 36 months (n = 54).Data are presented as the average differences of the TWSTRS score.
Discussion
The INTEREST IN CD2 study represents an international effort to describe satisfaction regarding BoNT-A and clinical effects through time in a non-controlled setting, suggesting that patient satisfaction is relevant information to assess effectiveness9, and also quality of daily life10.
Regarding the proportion of patients who had previously been treated with BoNT-A, it was high, and considering an average of 7.3 months between the last treatment and enrollment, we could suggest that subjects continue to receive benefit from repeated and previous treatments. Although the proper identification of the dystonic muscles remains a challenge11, the principal injected muscles in this study remain similar to those most frequently described in the previous studies12.
Moreover, prescriptions of type of BoNT-A were similar to that reported in other real-life clinical management studies on other conditions10; where abobotulinumtoxin A was the most commonly used agent (84.8%), followed by onabotulinumtoxin A (2.5%) and incobotulinumtoxin A (1.3%). Likewise, many patients were receiving other therapies at the time of their BoNT-A treatment; it is possible that this could affect the main therapy; further information and analysis to elucidate their influence are needed.
This sub-analysis provides valuable information in terms of the evolution and attainment of longer-term treatment for CD through individual improvements and subjects' satisfaction in patients with CD given by the treatment over 36 months. Nine of each ten subjects were satisfied at the end of the follow-up, similar figures those reported in an international survey where 93.3% of patients with CD in Germany, France, USA, and Canada reported being very or somewhat satisfied after the first application of BoNT-A9. In addition, our figures in the proportion of patients that seemed to respond in a favorable way regarding satisfaction to the treatment (88%) at 3 years of follow-up, are slightly higher to the reported by Dressler (78.9%)13.
The authors recognize some limitations for this study, stating that even the sample represents two Latin American countries; the sample is not quite representative. However, the number of sites and mechanisms to recruit patients could provide an overview of the therapeutic management and the response of patients with CD. Besides, data obtained and interpreted from the Likert scale and TWSTRS to assess effectiveness and satisfaction is mainly based on the clinical experience14 and on the subjective value of the patient, so we considered this information could be enhanced with other instruments to get a better understanding of the impact of the treatment over other clinical variables as the duration of the effect, stability, reproducibility, discomfort15, or even being specific for body regions for which scales are not available yet16.
Conclusion
This study suggests that patients with CD under repeated treatment with BoNT-A present a constant improvement on satisfaction rate as well as a clinically significant effect response during a follow-up of 36 months in a setting close to real-life management. Furthermore, this study suggests the feasibility to international comparisons to demonstrate that continued treatments could lengthen in convalescences in clinical features and lead to remain symptoms relatively constant.
Disclosures
This study was sponsored by Ipsen Pharma. Dr. Hernández Franco J receives income and participate in academic activities by Allergan, Ipsen, and Merz; Dr. Rodriguez Violante MDJ receives income by Allergan, Medtronic, UCB, Vanquish, and Everneuropharms; Dr. López Ruiz M has research studies with Merck, Sanofi, Pfizer, Biogen, and Roche; Dr. Hernandez Gomez JFJ receives funds for sponsorships or research, fees, and collaboration in practical theoretical workshops; Dr. Espinosa Sierra L, Dr. Quiñones Aguilar S, and Dr. Quiñones Canales G, they currently have no job with another company.
This study was sponsored by Ipsen Pharma. INTEREST IN CD2 study group: Dr. Espinosa Sierra L, Dr. Hernandez Gomez JFJ, Dr. Hernández Franco J, Dr. López Ruiz M, Dr. Rodriguez Violante MDJ, Dr. Quiñones Aguilar S, and Dr. Quiñones Canales G.











 nueva página del texto (beta)
nueva página del texto (beta)


