Introduction
Symptomatic palatal tremor (SPT) is an uncommon hyperkinetic movement disorder1. The pathophysiological basis of SPT is hypertrophic olivary degeneration (HOD), causing interruption of the dentato-rubro-olivary pathway due to lesions in the olivary body at the medulla oblongata. These lesions usually affect the Guillain–Mollaret triangle (GMT) formed by the red nucleus, the inferior olivary nucleus (ION), and the dentate nucleus2. The interruption of the cerebellar inhibitory output to the ION gradually causes hypertrophy, leading to abnormal neuronal discharges and subsequent tremor3. In SPT, the levator veli palatini is the main affected muscle, causing a 1-2 Hz palatal tremor that usually persists during sleep; other signs such as ocular, pharyngeal or rubral tremor, ataxia, and pendular nystagmus can also be present4,5.
Although sometimes considered myoclonus, its rhythmic nature and bilateral, symmetrical affection of the soft palate and pharynx is consistent with a tremor. Rhythmic myoclonus is often slower than tremor, is present at rest, is not modified significantly by voluntary movements, and often persists during sleep6,7.
The average reported time between an initial GMT lesion and SPT development is 10-11 months8.
SPT reports in the past have described it as a medical curiosity, but this could be due to a low level of diagnostic suspicion and late-onset appearance8,9.
Methods
We describe a large cohort of consecutive patients with SPT who were assessed by the same neurologist in a tertiary referral center, including clinical findings, diagnostic workup, and treatment. Subsequent patients > 18 years with an oscillatory palatal tremor of 1-2 Hz and history of posterior fossa disease were included. All patients included were evaluated for treatment response with a mean follow-up of 11 months.
The study was approved by the local ethical committee and classified as a non-risk study with no intervention. Clinical, images, or video film records were obtained from corresponding patient authorization by written consent in all cases.
Results
A total of 27 patients were included in this cohort from 1998 to 2016; 16 males and 11 females, with a mean age of 47 (range 19-89 years). The average time from presentation to the diagnosis of SPT was 14.5 months (range 2-48 months). The main etiology of SPT was cerebrovascular disease (CVD) with 21 (78%) patients, of which 14 (66%) were classified as ischemic. There were 3 (11%) cases related to infectious diseases: one case due to neurocysticercosis of the fourth ventricle and two others secondary to cerebral toxoplasmosis (in the context of HIV infection) of the posterior fossa. Two females (7%) had brainstem lesions due to neuromyelitis optica spectrum disorder (NMOSD). The remaining case was a young man with progressive ataxia with a probable neurodegenerative disease. Inferior olivary degeneration (T2 sequence hyperintensity on magnetic resonance imaging [MRI]) with or without hypertrophy was observed in 13 of 14 (93%) patients who had available MRI performed at follow-up (Figs 1-3). Except for the patient with progressive ataxia, all other 26 patients had a history of injury to the posterior fossa, in addition to rhombencephalic signs, including severe spastic or ataxic dysarthria. None of the patients responded significantly to pharmacological treatment during the follow-up (specific treatment is described in Table 1).
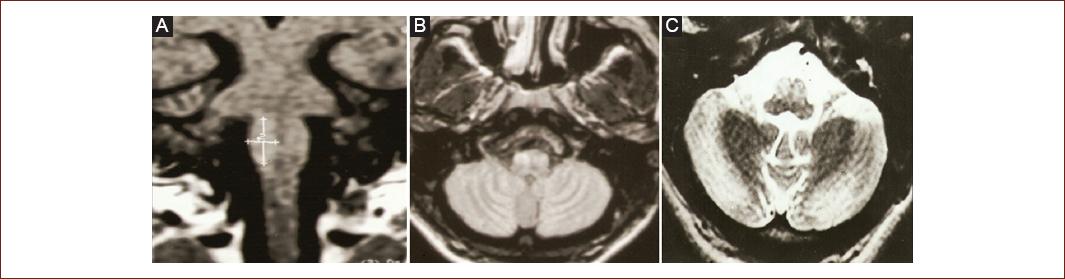
Figure 1 (Patient 2) Magnetic resonance imaging. Coronal T1 A: axial FLAIR B: and axial T2 C: weighted imaging showing olivary hypertrophy degeneration in a patient with progressive ataxia.
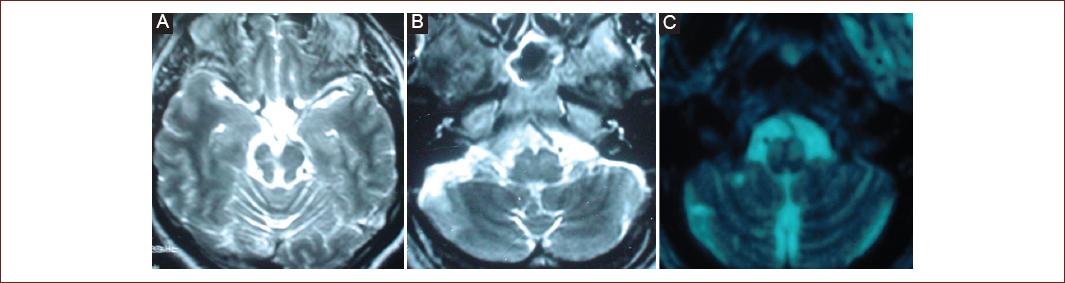
Figure 2 (Patient 18) Magnetic resonance imaging. Axial T2-weighted imaging A-C: showing multiple, chronic infarctions in the vertebrobasilar territory (cerebellum and mesencephalon), and olivary hypertrophy.
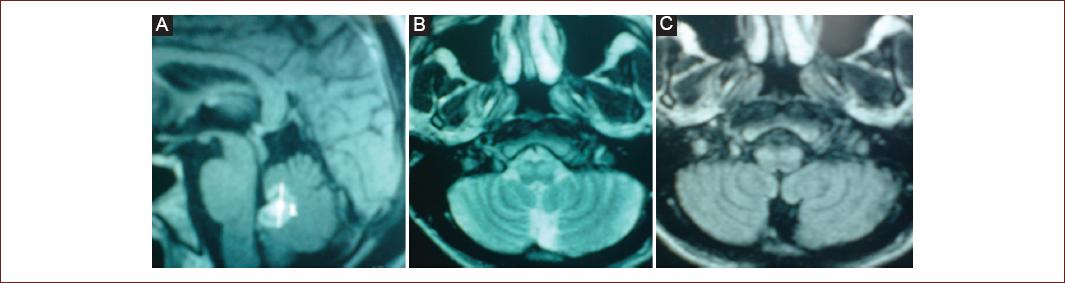
Figure 3 (Patient 20) Magnetic resonance imaging (MRI). Ring enhancement on sagittal T1 contrast-enhanced weighted imaging in the fourth ventricle A: consistent with toxoplasmosis in a patient with HIV, axial T2 B: and FLAIR C: with olivary hypertrophy 11 months after follow-up.
Table 1 Symptomatic palatal tremor etiologies, time to diagnosis, treatment, outcome, and MRI findings
| No. | Sex/age | Etiology (initial neurological insult) | Time to diagnosis (months) | Treatment | Follow-up (months) | Palatal tremor outcome | MRI inferior olivary hyperintensity/ hypertrophy |
|---|---|---|---|---|---|---|---|
| 1 | M/48 | Cerebellar infarct | 24 | Propranolol | 4 | Unsuccessful | Yes |
| 2 | M/34 | Progressive ataxia | 11 | Clonazepam/botulinum toxin | 6 | Decrease in frequency | Yes |
| 3 | M/25 | Multiple infarcts in vertebrobasilar territory | 9 | None | 8 | Unsuccessful | Yes |
| 4 | F/25 | Multiple infarcts in vertebrobasilar territory | 12 | Clonazepam | 10 | Unsuccessful | Yes |
| 5 | F/47 | Right cerebellar hypertensive hemorrhage | 36 | None | 36 | Unsuccessful | Yes |
| 6 | M/41 | Cerebellar peduncular hemorrhage | 13 | Propranolol | 4 | Unsuccessful | Unknown |
| 7 | F/75 | Mesencephalic infarct | 8 | Propranolol/levetiracetam | 14 | Unsuccessful | Unknown |
| 8 | M/89 | Cerebellar infarct | 9 | Propranolol/levetiracetam | 2 | Unsuccessful | Unknown |
| 9 | M/24 | Multiple infarcts in vertebrobasilar territory | 10 | None | 3 | Unsuccessful | Unknown |
| 10 | M/64 | Cerebellar hypertensive hemorrhage | 24 | Propranolol/levetiracetam | 13 | Unsuccessful | Yes |
| 11 | M/28 | Multiple infarcts in vertebrobasilar territory | 12 | Acetazolamide/levetiracetam | 25 | Decrease in frequency | Yes |
| 12 | F/20 | Cerebellar hemorrhage | 11 | Propranolol | 8 | Unsuccessful | Unknown |
| 13 | F/34 | Fourth ventricle neurocysticercosis | 48 | Propranolol | 1 | Unsuccessful | Unknown |
| 14 | M/46 | Multiple infarcts in vertebrobasilar territory | 17 | None | 4 | Unsuccessful | Unknown |
| 15 | F/19 | Thalamomesencephalic hemorrhage | 29 | Propranolol | 8 | Speech improvement | No |
| 16 | M/78 | Cerebellar infarct | 8 | Propranolol | 9 | Unsuccessful | Unknown |
| 17 | M/70 | Pontine hypertensive hemorrhage | 5 | Propranolol | 5 | Unsuccessful | Unknown |
| 18 | M/43 | Multiple infarcts in vertebrobasilar territory | 12 | Propranolol | 19 | Unsuccessful | Yes |
| 19 | F/61 | Multiple infarcts in vertebrobasilar territory | 2 | Propranolol | 9 | Unsuccessful | Unknown |
| 20 | M/35 | Toxoplasmosis of cerebellum and roof of fourth ventricle | 11 | Propranolol | 7 | Unsuccessful | Yes |
| 21 | M/56 | Multiple infarcts in vertebrobasilar territory | 6 | None | 5 | Unsuccessful | Unknown |
| 22 | F/68 | Multiple infarcts in vertebrobasilar territory | 10 | Propranolol /levodopa /biperidene | 8 | Unsuccessful | Unknown |
| 23 | M/56 | Multiple infarcts in vertebrobasilar territory | 18 | Nifedipine | 3 | Unsuccessful | Unknown |
| 24 | F/56 | Brainstem and diencephalic hemorrhage | 16 | Propranolol Levetiracetam | 16 | Unsuccessful | Yes |
| 25 | F/48 | Neuromyelitis optica spectrum disorder | 12 | None | 38 | Unsuccessful | Yes |
| 26 | F/63 | Neuromyelitis optica spectrum disorder | 9 | Carbamazepine | 16 | Unsuccessful | Yes |
| 27 | M/40 | Toxoplasmosis in posterior fossa (HIV +) | 12 | None | 8 | Unsuccessful | Yes |
M: male; F: female.
Discussion
Since its first report by Oppenheim in 1887, HOD continues to be an uncommon condition with a variety of etiologies and clinical manifestations, with palatal tremor as a unifying criterion10.
In this study spanning almost two decades, our neurology staff was able to identify approximately 1.5 new cases of SPT per year using the following approach: a history of posterior fossa disease, a careful physical examination, and ordering appropriate neuroimaging studies; making SPT a more common condition than previously reported6,7. Patients with a history of posterior fossa surgery were not included in our registry, making this condition probably more frequent in neurosurgery departments. Clinically, the HOD can also cause dentato-rubral tremor (Holmes tremor) and ocular myoclonus as late-onset manifestations; however, these symptoms did not occur in any of our patients during follow-up2,11.
In our study, the most common cause of SPT was brainstem or cerebellar CVD, representing almost 80% of reported cases. These data are consistent with results from other studies, in which the main causes of SPT were CVD, trauma, metastatic, and astrocytic tumors, multiple sclerosis, surgical interventions, syringobulbia, Behçet’s disease, and encephalitis12-14.
Although most of the patients with HOD and SPT have no symptomatic discomfort, several drugs, including beta-blockers, antiepileptic, benzodiazepines, and neuroleptics, are commonly used12. They usually offer partial and non-sustained symptom control in most cases, as reported in this study. Some recent models have demonstrated the influence of cerebellar Purkinje-cell modulation to the hypertrophic ION in SPT, suggesting drug combination therapy for a “double” inhibition with more satisfactory results. These therapies include the use of drugs with GABA-enhancing inhibitory and glutamatergic-coupling effects15.
Other non-pharmacological options include the administration of botulinum toxin, a safe and effective therapy in selected patients with SPT, and marginal benefits using special dental devices that have been used by some authors in patients with lingual-palatal tremor16-18.
Deep brain stimulation has not been studied in SPT but is a secure and beneficial procedure in other movement disorders such as essential tremor and Parkinson’s disease. It could provide some benefit for SPT in the future19,20.
Patients with a history of brainstem or cerebellar injury with no palatal tremor at diagnosis should be kept under vigilance since SPT can appear during follow-up, up to 30 months after initial presentation. Patients with an incidental finding of palatal tremor should be evaluated to rule out lesions in the posterior fossa since over 75% of cases reveal an underlying symptomatic cause21. This could be a typical scenario of patients with undiagnosed multiple sclerosis or NMOSDs who initially present with posterior fossa symptoms and visit another specialist (such as an otorhinolaryngologist) for medical advice.
During the study, we have been able to identify three clinical characteristics that may facilitate and increase a diagnosis of SPT: (1) history of posterior fossa disease; (2) persistence of severe rhombencephalic signs during the 1st year of symptom onset; and (3) predominance of severe spastic or ataxic dysarthria. More prospective studies with consecutive clinical evaluations should be done to determine the predictive factors involved in these reported findings.











 nueva página del texto (beta)
nueva página del texto (beta)


