Introduction
The Coronaviridae family is comprised by enveloped positive-sense single-stranded RNA viruses, some of which are capable of causing human infection. These include several alpha-coronaviruses (human coronavirus [HCoV]-NL63 and HCoV-229E) and beta-coronaviruses (HCoV-HKU1, HCoV-OC43, Middle East respiratory syndrome-CoV [MERS-CoV], and severe acute respiratory syndrome [SARS-CoV])1. In December 2019, a highly pathogenic coronavirus (CoV) emerged in Wuhan, China and rapidly spread around the world2,3. Initially, this pathogen was named as 2019 novel CoV (2019-nCoV) by the World Health Organization. After a similar viral structure and infection pathway was discovered between this novel virus and SARS-CoV, the official name was changed to SARS-CoV-24,5. The diseases caused by this nCoV are mostly related to the respiratory system and are named CoV disease 2019 (COVID-19)5.
Even though the most widely recognized organ affected by CoVs is the respiratory system, it is important to note that CoV are not exclusively confined to the respiratory tract6. In fact, the neuroinvasive ability of this pathogen has been documented for almost all beta-coronaviruses in animal (mouse hepatitis virus [MHV] and porcine hemagglutinating encephalitis virus) and human (HCoV-229E, HCoV-OC43, SARS-CoV, and MERS-CoV) hosts1,7,8. Because of the high similarity between SARS-CoV and the novel SARS-CoV-2, it is likely that this new pathogen has analogous neuroinvasive properties. Moreover, it has been described that 36-84% of hospitalized patients with COVID-19 manifest neurological symptoms, underscoring the potential nervous system involvement by this disease9,10. Thus, it is important to examine the possible routes of infection by this pathogen and the neurological manifestations that patients affected by this disease might express.
Routes for nervous system involvement by SARS-CoV-2
The main mode of human-to-human transmission of the SARS-CoV-2 infection is presumed to be by respiratory droplets, which allows the virus to come into contact with mucosal epithelium (e.g., nasal mucosa) and invade susceptible cells11. Specifically, it has been demonstrated that this virus exhibits a high affinity to the angiotensin-converting enzyme 2, a cellular receptor that is ubiquitously expressed by respiratory epithelium, lung parenchyma, vascular endothelium renal tissue, small intestine cells, neurons and glial cells, among others5,6.
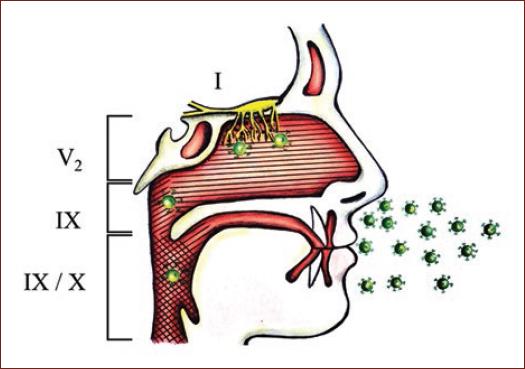
Experimental models using transgenic mice have revealed that intranasal CoV inoculation could result in central nervous system (CNS) infection through retrograde axonal transport from olfactory nerves12,13. Hence, SARS-CoV-2 present in nasopharyngeal structures could access the brain similarly by invading local cranial nerves (i.e., olfactory, trigeminal, glossopharyngeal, and vagus nerves) (Fig. 1). Furthermore, it has been postulated that other potential pathways of invasion in patients with this disease is through the enteric nervous system or the hematogenous route8,14. Specifically, viremia could result in subsequent invasion of the CNS by infected leukocytes or through the endothelium of the blood-brain barrier1. In addition, circumventricular organs as well as dorsal root and autonomic ganglia which lack a blood-nerve barrier could act as potential hematogenous routes for pathogen entry into the CNS15,16. Thus, considering that multiple pathways for neurological invasion of the CNS exist, neurological manifestations of COVID-19 could be more frequent than initially presumed.
Potential mechanisms of neurological manifestations of SARS-CoV-2
A variety of neurological manifestations have been attributed to SARS-CoV-2 infection, ranging from headache to acute cerebrovascular events17. The mechanism of the neurological disease exhibited by COVID-19 patients might be a consequence of direct viral invasion of the nervous system, indirect CNS injury by abolition of systemic homeostasis, or a combination of both4-6. However, how to differentiate if a manifestation of the disease is a consequence of direct damage to the nervous system or evidence of systemic derangements is currently unclear.
Notably, the CoVs have been shown to mediate nervous system damage through virus-mediated injury to neurons and glial cells, as well as through damage mediated by the activation of the immune system8. On the other hand, indirect neurological manifestations can occur when there is a widespread dysregulation of homeostasis secondary to multiple organ involvement. It is now evident that SARS-CoV-2 can precipitate multiple organ failure, shock, heart failure, arrhythmias, and renal injury in addition to pneumonia and acute respiratory distress syndrome18. These presentations might be secondary to hypoxia, hypercoagulability, and release of excessive pro-inflammatory cytokines, which have the potential to indirectly cause neurological symptoms without CNS invasion by the virus19,20. Furthermore, the release of cytokines caused by viral infection could result in the permeabilization of the blood-brain barrier, allowing the invasion of the CNS by T lymphocytes and resulting in neuroinflammation21,22. Such disruption of the blood-brain barrier could facilitate the entry of SARS-CoV-2 through the hematogenous route, thus resulting in concurrent direct and indirect damage of the CNS mediated by this pathogen22.
To elucidate the nervous pathophysiology of SARS-CoV-2 infection, it has been proposed that direct invasion of the CNS can be supported by the presence of viral RNA in cerebrospinal fluid (CSF). Nonetheless, failure to detect virus in the CSF does not exclude invasion of the CNS as illustrated in a case report by Paniz-Mondolfi et al.23. Consequently, further research is needed to determine which markers can be useful to distinguish between the direct involvement of the nervous system by SARS-CoV-2 and nervous injury secondary to systemic inflammation.
Reports of neurological manifestations in COVID-19 patients
Two large retrospective case series have examined the prevalence of neurological manifestations in patients hospitalized with COVID-19. Mao et al9. analyzed data from three centers located in Wuhan, China, including a total of 214 hospitalized COVID-19 patients. They found that 36.4% of their cohort exhibited neurological symptoms: 36 (16.8%) manifested dizziness, 28 (13.1%) headache, 23 (10.7%) skeletal muscle injury, 16 (7.5%) altered mental status, 12 (5.6%) hypogeusia, 11 (5.1%) hyposmia, 6 (2.8%) acute cerebrovascular disease, 5 (2.3%) neuralgia, 3 (1.4%) vision impairment, 1 (0.5%) ataxia, and 1 (0.5%) seizures. On the other hand, Helms et al10. reported the findings of 58 COVID-19 patients admitted to two intensive care units located in Strasbourg, France. They discovered neurological signs in 49 (84%) of their cohort: 40/58 (69%) presented agitation, 39/58 (67%) corticospinal tract signs, 26/40 (65%) confusion, 14/39 (36%) dysexecutive syndrome, and 8/49 (16%) fever > 38.5°C. Of the 13 patients that had a brain MRI because of unexplained encephalopathic features, 11/11 (100%) presented perfusion abnormalities, 8 (62%) leptomeningeal enhancement, and 3 (23%) cerebral ischemic stroke. CSF samples were analyzed for seven cases, all of which had a negative reverse transcription-polymerase chain reaction for SARS-CoV-2, which suggests that the neurologic clinical findings could have been secondary to indirect CNS injury or toxic encephalopathy.
In addition to the aforementioned studies, the study performed by Li et al24. merits mention as they assessed the prevalence of cerebrovascular disease in hospitalized patients with COVID-19. In 211 consecutive patients were admitted to a center in Wuhan China, 11 (5%) developed acute cerebrovascular ischemic stroke, 1 (0.5%) exhibited cerebral venous sinus thrombosis, and 1 (0.5%) presented with intracerebral hemorrhage.
Several authors have published case-series and case reports of neurological manifestations of COVID-19. These reports comprise patients aged from 24 to 79 years presenting with a variety of symptoms such as headache, altered mental status, seizures, neck rigidity, muscle weakness, incontinence, and corticospinal tract signs. The neurological diagnosis established in such cases include Guillain-Barre syndrome25,26, encephalopathy27,28, meningoencephalitis2, acute myelitis29, and intracerebral hemorrhage30.
Noteworthy, another neurological manifestation of COVID-19 that has been proposed is neurogenic respiratory failure, which together with diffuse alveolar damage, results in difficult to treat hypoxia characteristic of severe COVID-19 cases. In particular, several authors have advocated for brain stem dysfunction caused by direct viral invasion as a possible cause for respiratory and cardiovascular derangements observed in SARS-CoV-2 infection4,15,31.
In summary, the amount of evidence available indicates that COVID-19 is irrefutably associated with neurologic manifestations. However, to what extent these manifestations are a consequence of direct viral mediated damage has not been elucidated.
Potential long-term repercussions of COVID-19 infection
It has been previously described that CoVs have the potential to produce chronic infections in the CNS7,8. This can have significant clinical implications as the persistence of the viral infection could be associated with long-term neurological sequela and predispose to the development or progression of neurodegenerative and demyelinating disorders.
To date, there is no definitive evidence that links human infection by CoV to the development of chronic neurological diseases. Nonetheless, the presence of HCoV-229E and HCoV-OC43 RNA has been demonstrated in the CNS of patients with Parkinson's disease, Alzheimer's disease, multiple sclerosis (MS), and acute disseminated encephalomyelitis8. Particularly, the proportion of patients with detectable HCoV-OC43 RNA in brain parenchyma of patients with MS has been shown to be statistically significantly increased compared to healthy controls32. While it remains to be elucidated if persistent CoV infection plays a role in the pathogenesis of MS in humans, murine models have demonstrated that chronic MHV infection can induce immune-mediated demyelination7. Furthermore, persistence of HCoV-OC43 RNA in CNS of mice was associated with neuronal degeneration with secondary motor deficits appearing several months after acute infection by intracerebral inoculation, supporting the pathogenic role of chronic CoV infection33.
Of note, even in the absence of chronic presence of SARS-CoV-2 in the CNS, COVID-19 infection may have long-term neurologic sequela as a consequence of microglial priming and astrogliosis. These cells might become chronically active and release pro-inflammatory cytokines even after the initial stimulus for the inflammatory response has been eliminated, possibly leading to chronic neuroinflammation even in the absence of active infection22,31.
Consequently, as no information is currently available about the ability (of lack thereof) of SARS-CoV-2 infection to persist in CNS and mediate chronic neuronal damage, its potential to cause long-term sequela should not be dismissed.
Conclusion
Infection by SARS-CoV-2 might be associated with significant nervous involvement. However, the specific pathways for nervous system invasion and the mechanisms for neurological disease are currently unknown. As the magnitude of the acute neurological manifestations and the potential long-term repercussions of this disease are unclear, future efforts should concentrate on elucidating the pathophysiology of the neurological component of COVID-19.











 nueva página del texto (beta)
nueva página del texto (beta)