Introduction
In December 2019, a new virus belonging to the Coronavirus (CoV) family broke in as a pathogen in China's Wuhan region causing high-lethal severe acute respiratory syndrome (SARS). The World Health Organization (WHO) gave it the name SARS-CoV-2 given its similarity virologically and also in its clinical expression with SARS-CoV (229E [HCoV-229E]), responsible for a syndrome of similar characteristics also originating in animal markets in China in 20031. As of 24 February 2020, more than 80,000 confirmed cases have been reported, including more than 2700 deaths worldwide, affecting at least 37 countries. The WHO declared it a global health emergency by the end of January 20202.
CoVs are members of the family Coronaviridae, wrapped viruses that possess extraordinarily large single-chain RNA genomes ranging from 26 to 32 kb in length3. An unknown that continues to be investigated is the recognition of the zoonotic origin of said virus, but due to its close similarity to bat CoVs, it is likely that these are the primary reservoir of the virus, since with the reappearance of this new class of CoV various studies was performed and SARS-CoV-2 was found to be 96% identical at the genome level to a bat CoV; the same study revealed that the virus belongs to the SARS-CoV species. This is how SARS-CoV is speculated to have been transmitted to humans from exotic animals in markets in the outbreak 18 years ago, while Middle East respiratory syndrome coronavirus (MERS-CoV) was transmitted from camels to humans4.
Transmission from one person to another has been demonstrated, and the transmission mechanism is known to be by respiratory drops generated during coughs and sneezes by symptomatic patients, but may also occur by asymptomatic individuals and before symptoms onset5. Anderson et al. suggest transmission through aerosols predominantly < 1 mm, produced when speaking or with normal breathing by asymptomatic patients. It also suggests that aerosols may be a long-distance transmission vehicle due to suspension of aerosols in the air for several hours. All of the above related to survival time and distances, virus concentrations, effects of temperature and humidity, and the implications of aerosol size and viral load in contact with the respiratory tract6. Chan et al. report findings that suggest the transmission of person to person and that intercity spread of SARS-CoV-2 by air is possible7.
In a cohort study conducted by Li et al.8, SARS-CoV-2 has been reported in semen of the 38 patients undergoing the study8. Fecal-oral transmission has also been suggested and a vertical transmission mechanism has recently been proposed9.
SARS-CoV-2 uses the angiotensin-converting enzyme (ACE2) as its main receptor, which is widely expressed in vascular endothelium, respiratory epithelium, alveolar monocytes, macrophages, and neurons (Fig. 1). Later in the course of the disease, COVID-19 resembles SARS in terms of viral replication in the lower respiratory tract, and generates secondary viremia, followed by an extensive attack on target organs expressing ECA2, such as the heart, kidney, gastrointestinal tract, and vast distal vasculature10.
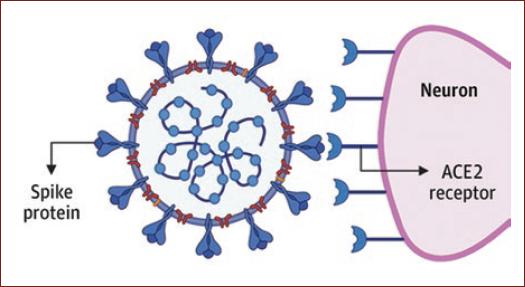
Figure 1 Angiotensin-converting enzyme (ACE)-2 receptors at a medullary junction to the SPIKE protein in severe acute respiratory syndrome coronavirus 2. Emerging data suggest that ACE 2 receptors are expressed in multiple regions of the human brain, including the motor cortex, posterior cingulate cortex, ventricles, Nigri substance, olfactory bulb, medium temporal turn, ventrolateral marrow, solitary tract nucleus, and vagus dorsal motor nucleus. (taken from Zubair et al.28).
The clinical characteristics of COVID-19 are varied, ranging from asymptomatic state to acute respiratory distress syndrome and multiorgan dysfunction11. Symptoms that have been reported in particular are fever, dry cough, dyspnea, myalgia and fatigue, less frequent confusion, headache, pharyngeal pain, rhinorrhea, abdominal pain, diarrhea, nausea, and vomiting9.
CoV is one of the main viruses that mainly affects the human respiratory system, but it also has neuroinvasive capabilities and can spread from the respiratory tract to the central nervous system (CNS)12.
In January 2020, Chen et al. published a retrospective analysis based on 99 patients diagnosed with SARS-CoV-2 pneumonia at a hospital in Wuhan, China. Neurological symptoms presented were confusion (9%) and headache (8%)13. In addition, different studies have been reported that assert neurological manifestations secondary to SARS-CoV-2, so attention should be paid to these manifestations because they may affect the prognosis and evolution of the patient.
Neurotropism's background
It is currently known that CoVs are not always limited to the respiratory tract and that they can also invade CNS by inducing neurological diseases. This neuroinvasive capacity of CoVs has been documented almost for all COVs, including SARS-CoV, MERS-CoV, HCoV-229E, HCoV-OC43, mouse hepatitis virus, and porcine hemagglutinating encephalomyelitis14.
The SARS pandemic that occurred in 2002 detected sequences of the virus genome in the brain of all SARS deceased autopsies with light microscopy, electron microscopy, and real-time reverse transcription-polymerase chain reaction (rRT-PCR). The signals were limited to the cytoplasm of numerous neurons in the hypothalamus and cortex15. The edema and scattered red degeneration of neurons were present in the brains of six of the eight confirmed cases of SARS. Viral SARS sequences and pathological changes were not present in the brain of unconfirmed cases or control cases15. Likewise, patients with acute SARS-CoV disease have also demonstrated the presence of the virus in cerebrospinal fluid16.
Overall, neuroinvasive ability has been shown to be a common feature of COVs. Considering the high similarity between SARS-CoV and SARS-CoV-2, SARS-CoV-2 is also likely to have similar potential14.
The spread of SARS-CoV-2 in systemic circulation or through the cribriform plaque of the ethmoid bone during an early or later stage of infection can lead to brain involvement as has been reported in the past for SARS-CoV16. The latter can be supported by the fact that in the study of Mao et al., some patients had hyposmia17.
The presence of the COVID-19 virus in the general circulation allows it to, understandably, move to brain circulation where the slow flow of blood within microcirculation could be one of the factors that can facilitate the interaction of the spike protein (protein S) of the SARS-CoV-2 virus with ACE-2 receptors16. These receptors are known to be expressed in the lungs, heart, kidneys, intestines, brain, and testicles by converting these different organs into possible SARS-CoV-2 targets16.
CNS manifestations
Inespecific manifestations
SARS-CoV-2 has been shown to exhibit non-specific neurological manifestations, which are well unknown whether they can be caused directly or indirectly by the virus. In a study by Mao et al., 214 patients, 88 (41.1%) and 126 (58.9%) they were not serious. Of these, 78 (36.4%) neurological manifestations involve CNS, peripheral nervous system, and skeletal muscles. Severe patients were more likely to develop neurological symptoms, especially acute cerebrovascular disease, altered consciousness, and muscle injuries17,18. It should be emphasized that headache is the most common neurological symptom in Wuhan, China according to study conducted by Guan et al.19.
Furthermore, in a study conducted in two different intensive care unit (ICU) in France, 58 patients reported neurological findings; agitation was present in 40 patients (69%). A total of 26 out of 40 patients were observed to have confusion under the ICU Confusion Assessment Method. Diffuse signs of the corticoespinal tract such as muscle stretching hyperreflexia, aquileo clonus, and bilateral Babinski sign were present in 39 patients (67%). Of the patients who had been discharged, 15 out of 45 (33%) had a dysexecutive syndrome that consisted of inattention, disorientation, or poorly organized movements in response to the command20.
These neurological manifestations have also been reported in Latin America. In Chile, a study was conducted with 922 positive cases (Table 1), of which 597 (64.8%) headache as a cardinal symptom, while only 8.5% and 49.0% had fever and cough, respectively, among other symptoms. The presence of headache in most of these patients may suggest the potential neurotropism and neurovirulence of SARS-CoV-2 as seen in other naturally neurotropic human CoVs such as SARS-CoV and HCoV-OC43 and −229E21.
Table 1 Main clinical findings in the first 922 cases of COVID 19 in Chile
| Síntomas | n | % |
|---|---|---|
| Headache | 597 | 64.8 |
| Dyspnea | 498 | 54.0 |
| Cough | 452 | 49.0 |
| Thoracic pain | 407 | 44.1 |
| Fever | 78 | 8.5 |
| Abdominal pain | 41 | 4.4 |
| Myalgias | 32 | 3.5 |
Adapted from Rodriguez-Morales et al., 202021.
Several observational studies have looked at symptoms of the disease, but few have addressed neurological symptoms that go beyond headache and confusion22.
Meningitis and encephalitis
The first case of aseptic encephalitis was reported in a 24-year-old patient, brought to the emergency by an ambulance due to a seizure accompanied by unconsciousness, presenting multiple generalized tonic-clonic epileptic seizures, and obvious neck stiffness. It was taken both nasopharyngeal and cerebral spinal fluid (CSF) samples and was diagnosed using RT-PCR technique by finding SARS-CoV-2 RNA in cerebrospinal fluid23.
Interestingly, imaging findings were also found in this patient, reporting a brain magnetic resonance imaging (MRI) with hyperintensity along the wall of the right lateral ventricle and hyperintense signal changes in the right mesial temporal lobe and hippocampus, suggesting the possibility of SARS-CoV-2 meningitis23.
In the current pandemic, Xiang et al.'s team, the Beijing Ditan Hospital confirmed the presence of SARS-CoV-2 virus in cerebrospinal fluid in patients positive for COVID-19. The finding was made using genomic sequencing techniques, as well as clinically confirming viral encephalitis in patients. This provides a solid foundation for considering CoVs as causing encephalitis18.
Positive COVID-19 patients have been reported, which usually present a clinical picture of meningitis/encephalitis, but with CSF RT-PCR tests for SARS-CoV-2 negative. Some authors argue that this happens because of the low inoculum that can occur in the CSF.
This is the case of a 64-year-old patient, who at the neurological examination indicated that the patient was impaired in mental state, with an alternating consciousness between lethargy and irritability. Both lower limbs showed positive ankle clonus, which was more pronounced in the left limb. The lower left limb was positive for Babinski's sign and Chaddock's sign; the lower right limb was suspected positive for Chaddock's sign. He also showed meningeous signs24.
There is also a positive patient for COVID-19 who has impaired consciousness accompanied by marked meningeous signs and Babinski's sign. After a thorough neurological evaluation by experts, the diagnosis of SARS-associated encephalitis CoV-2 was established, even though the CSF sample test tested negative for SARS-CoV-225.
Encephalopathy
Encephalopathy is a transient brain dysfunction syndrome that manifests as an acute or subacute affectation of the level of consciousness. COVID-19-associated encephalopathy may be due to toxic and metabolic causes, and the effect of hypoxia or drugs. Another associated indirect mechanism is the presence of subclinical crises26. Encephalopathy is a demonstration of how CNS circuits responsible for arousal, perception and focus are distributed and how are susceptible to infectious, toxic, or metabolic systemic disorders. The CNS's response to such disorders is often relatively acute, and a variety of different irritants often produce the same nonspecific behavioral reactions27.
Elderly patients with chronic diseases have an increased risk of mental state disturbance in the acute infection environment. One of the possible etiological mechanisms of encephalopathy could be due to the cytokine storm which along with other comorbidities and risk factors contributes significantly to toxic metabolic encephalopathy in severe cases28.
Mehta et al.29 describes a cytokine profile associated with the severity of COVID-19, characterized by an increase in interleukin (IL)-2, IL-6, IL-7, granulocyte colony stimulating factor, interferon-gamma inducible protein 10, 1-necroattract monocyte protein, 1-alpha inflammatory macrophage protein, and necrosis tumor factor. This cytokine storm in combination with different comorbidities and metabolic imbalances could be the cause of encephalopathy.
Because COVID-19 affects more elderly and those with pre-existing conditions, patients with the previous neurological conditions and acute respiratory symptoms have an increased risk of encephalopathy at baseline17,30. One study reported an electroencephalogram that showed diffuse slow waves in the bilateral temporal region in the patient's studied30.
Acute necrotizing encephalopathy (ANE)
A case of ANE hemorrhagic has also been reported in a patient diagnosed with COVID-19 who had symptoms of fever, cough, and altered mental state. Diagnosis was performed by detection of SARS-CoV-2 by PCR-TR in a nasopharyngeal sample. Brain computed tomography (CT) detected a symmetrical, bilateral hypodense area in the medial thalamic nucleus. The MRI showed hemorrhagic lesions that enhanced after contrast, multifocal and symmetrical disposition, in annular form in both thalamus, insula, and the medial region of the temporal lobes31.
ANE is a rare complication of influenza and other infections and has been related to intracranial cytokine storms, which result in decay of the blood-brain barrier, but without direct viral invasion or parainfectious demyelination32. Accumulated evidence suggests that a subgroup of severe patients with COVID-19 may have cytokine storm syndrome29.
Cerebrovascular disease
The mechanism by which SARS-CoV-2 virus enters the system by binding the S protein to ACE 2 receptors is known. ACE 2 through a signaling mechanism lowers blood pressure. Since the expression of these receptors is decreased in hypertensive patients, the ability of ACE2 to reduce blood pressure is affected in these patients. After SARS-CoV-2 infection, the expression and function of ACE2 proteins are reduced33. As a second line of evidence suggesting that SARS-CoV-2 infection may induce brain hemorrhage, patients with COVID-19 often suffer from coagulopathy and prolonged prothrombin time, which also contributes to secondary brain haemorrhage33.
In addition, patients in critical condition with severe SARS-CoV-2 infections often show elevated levels of D-dimer34 and severe reduction of platelets, which can make these patients prone to acute cerebrovascular events15.
In a retrospective study in Wuhan, China, it was reported that 13 out of 221 COVID-19 patients developed CVD after infection; 11 (5%) developed acute ischemic stroke, 1 (0-5%) cerebral venous sinus thrombosis, and 1 (0-5%) brain hemorrhage. Patients with CVD had elderly and cardiovascular and cerebrovascular risk factors. Importantly, 11 of the 13 CVD were severe patients with SARS-CoV-2 infection, suggesting that severe infection may be an indicator of CVD, especially acute ischemic stroke. Older patients with COVID-19 may be more likely to develop CVD and more attention should be paid to older patients with cerebrovascular risk factors. Mortality was 38%35.
The Mao et al. series describes five patients with stroke (80% ischemic), who had severe forms of COVID-19, with increased levels of D-dimer, thrombocytopenia, and multiple organ involvement17.
Oxley et al. report the case of five patients under the age of 50, positive for COVID-19 in New York City, who suffered an ischemic cerebrovascular event36. Some have symptoms such as impaired consciousness, lethargy, and headache. All patients reported involvement of large brain vessels such as the internal carotid artery, the middle cerebral artery, and the posterior cerebral artery.
Anosmia, hyposmia, and dysgeusia
Although SARS-CoV-2 has many similarities to SARS-CoV and MERS, clinical data reveal differential characteristics, including olfactory alterations and hallucinations; these symptoms have not been reported in patients with SARS-CoV or MERS-CoV infection22.
Several countries, including Germany, the United Kingdom, and Italy, have reported an increasing number of neurological manifestations such as anosmia (loss of the ability to detect one or more odors, temporary, or permanent), hyposmia (decreased sensitivity to some or all odors), ageusia (loss of taste functions), dysgeusia, or parageusia (the distortion of taste), and hypogeusia (decreased sensitivity to taste) in patients with confirmed SARS-CoV-2 infection21.
The mechanism of neuroinvasion through the cribriform plate to the olfactory nerve, besides from being an entry mechanism to the CNS, is believed to affect the sensory capacity of the olfactory nerve. A recent study showed that nasal epithelial cells show a very high expression of ACE 2 receptors37 within the SARS-CoV-2 infection, allowing a wide viral entry38. SARS-CoV has demonstrated, in a mouse model, transneuronal penetration through the olfactory bulb14,39.
In a European multicenter study conducted by Lechien et al. 357 patients (85.6%) out of a total of 417 patients interviewed, had infection-related olfactory dysfunction. Among them, 284 (79.6%) patient had anosmia and 73 (20.4%) were hypostomatic. Fantosmia and parosmia account for 12.6% and 32.4% of patients during the course of the disease, respectively40. In the above-mentioned study, taste disturbances have also been reported, a total of 342 patients (88.8%) reported taste disorders, which was characterized by the deterioration of the following four flavor modalities: salty, sweet, bitter, and acidic. Taste dysfunction consisted of a reduced/discontinued or distorted capacity to identify flavors in 78.9% and 21.1% of patients, respectively40.
The cause of dysgeusia is not yet very well established, however, in one study, a high expression of ACE 2 receptors was found in the epithelium of the tongue41. Animal studies show the expression of ACE 2 receptors in the solitary tract nucleus42 which could point to the central cause of dysgeusia and a possible neuroinvasive route by continuous retrograde axonal transport in humans43.
Periferic nervous system manifestations
Guillain-Barre syndrome (GBS)
The first case of GBS was reported, being a 61-year-old woman who had acute leg weakness and fatigue, which progressively advanced from the first day of initial symptoms. She returned from Wuhan on January 19, but denied having submitted COVID-19 compliant clinic. She was approached through a neurological examination that revealed symmetrical weakness (Medical Research Council Grade 4/5) and arreflexia in both legs and feet. Her symptoms progressed after 3 days of her admission. Muscle strength was 4/5 grade on both arms and hands and 3/5 on both legs and feet. CSF showed (5-10/L, normal: 0-8-10/L) and increased protein level (124 mg/dL, normal: 8-43 mg/dL). Nerve conduction studies (day 5) showed delayed distal latencies and absent F-waves in early course, supporting demyelinating neuropathy. On day 8 of disease evolution (January 30), the patient developed dry cough and a fever of 38.2°C. Chest CT showed ground-glass opacities in both lungs. Oropharyngeal swabs were positive for SARS-CoV-2 on RT-PCR assay. Furthermore, she was diagnosed with GBS and was given immunoglobulin intravenously44.
It is also the case of a 76-year-old woman, transferred to the Navarra Hospital Complex for presenting a 10-day picture of the evolution of lumbar pain radiated to the back of both legs and progressive tetraparesis with distal onset paresthesias. Eight days before the beginning of the clinical manifestation, he had started with cough and fever without dyspnea, 72 h of evolution, having been treated with amoxicillin-clavulanic and azithromycin. PCR test was performed for SARS-CoV-2 detection with a positive result45.
Even more characteristic cases of GBS have been reported, such as the report of a case of Miller Fisher syndrome and the case of a patient with multiple cranial neuropathies, both associated with COVID-1946.
Transverse myelitis
Transverse myelitis includes a pathologically heterogeneous syndrome characterized by acute or subacute spinal dysfunction resulting in paresis, a sensory level, and an autonomic impairment (bladder, bowel, and sexual impairment) below the level of the injury47.
A 66-year-old man with COVID-19 was admitted with acute flaccid bilateral lower extremity paralysis and urinary and intestinal incontinence. All serum microbiological test results were negative, except SARS-CoV-2 nucleic acid tests. Clinical findings indicated acute post-infectious myelitis. He was treated with ganciclovir, lopinavir/ritonavir, moxifloxacin, dexamethasone, human immunoglobulin, and mecobalamin. With a diagnosis of acute post-infectious myelitis and comprehensive treatment, bilateral lower limb paralysis improved48.
Discussion
SARS-CoV-2 has been shown not only to be an injury to the respiratory system, affectation of different systems but has also been reported, and one of the most affected is the nervous system. The mechanisms, direct or indirect, by which many of the above manifestations are caused by COVID-19, are unknown. There is evidence of a close relationship between the onset of neurological symptoms and a history of viral infection. General symptoms such as headache or a state of confusion in a confirmed COVID-19 patient should alert the clinician, and always keep in mind the likely onset of a manifestation or eventuality that compromises the patient's prognosis.
Detection and diagnosis of COVID-19 should be rapid and accurate to reduce the rate of contagion inside and outside hospital institutions. Specialists in the area of neurology are advised to be those patients with manifestations that guide a neurological pathology, to supplement the clinic with epidemiology in search of epidemiological links or risk factors in these patients, to establish the link between a neurological manifestation probably secondary to COVID-19. Due to the large percentage of asymptomatic patients today, it is recommended to perform current diagnostic tests such as RT-PCR, rRT-PCR, and reverse transcription loop mediated isothermal amplification49. Also take all biosecurity measures recommended by the Centers for Disease Control50.
Once the diagnosis is established, Natoli et al. recommend that a record containing epidemiological data should be taken into account in patients with neurological manifestation associated with COVID-19 to fully understand whether and to what extent SARS-CoV-2 infections may cause CNS involvement. Also measure the viral load of SARS-CoV-2 in CSF in symptomatic and asymptomatic patients, for comparison. Finally, conduct research through autopsies to develop a characterization and find a distribution of the virus in different tissues, in this way, the neuropathological consequences can be determined51.
Conclusion
SARS-CoV-2 has demonstrated great neuroinvasive ability. A neurological complication added to a disease such as COVID-19 can turn the patient's evolution and prognosis into something bleak, not to mention the possible consequences that it may leave. The mechanism by which many of the above manifestations are caused by COVID-19 is unknown, but there is evidence of a close relationship between the onset of neurological symptoms and a history of viral infection. Studies aimed at establishing physical mechanisms of these manifestations are recommended to improve both the patient's evolution and prognosis.











 nueva página del texto (beta)
nueva página del texto (beta)


