Introduction
Tacrolimus (FK-509 or fujimycin) is a macrolide inhibitor of calcineurin which limits the signaling of T lymphocytes and transcription of IL-2; therefore, it is a pillar in the treatment of immunosuppression after the transplant of solid organs worldwide, confirming the protocol Elite-Symphony in 20071.
The toxic effects related to calcineurin inhibitors are mostly metabolic (hypercholesterolemia and hyperglycemia), nephrotoxic, hematological, and neoplastic and infectious alterations.
Neurological complications have been the least studied despite presenting a relevant prevalence between 10 and 28%, containing a broad spectrum of manifestations ranging from mood changes to coma2.
In the review of the literature, there is limited information and not a lot of studies conducted to assess the neurological complications in this group. Muller et al.3 described post-operative neurological complications due to the use of tacrolimus in post-transplant liver patients.
It has been reported that the risk factors for developing neurological complications are hepatic failure, systemic hypertension, hypocholesterolemia, and high levels and concomitant use of methylprednisolone. However, it should be noted that many of the risk factors and complications present difficulties in establishing causality since many of these patients have multiple comorbidities and use other immunosuppressants2.
Although the association of neurological complications and tacrolimus is known, comparative studies between transplants of different organs in the long term are not yet available.
Tacrolimus has a great variability in its interindividual pharmacokinetics, so traditional dosing schemes are often obsolete and must be individualized from patient to patient. Therefore, constant monitoring of serum levels is important. It is known that reaching adequate serum levels is essential in the post-transplant period to avoid rejection, but it is still unknown at what speed and neurotoxic levels4.
The metabolism of tacrolimus is mainly hepatic in cytochrome P450 3A5, so alterations in this cytochrome can cause an erratic bioavailability, but it has been seen that the levels of hematocrit, weight, and use of steroids can also interfere5.
It has been observed that during the 1st year post-op, serum levels can change despite maintaining the same doses, therefore, up to 60% of patients will have non-therapeutic doses. Most physicians will establish the dose based on their clinical experience and will not use different tools designed for this purpose such as the use of specialized programs or measurement of CYP3A46.
Neurotoxicity
As previously mentioned, neurological complications can be found in up to 28% of the transplanted patients, but characteristics will differ depending on the transplanted organ and serum levels.
There is also a lack of evidence of the use of calcineurin inhibitors in the different transplanted organs, and therefore, a failure in the prevention of complications, in addition to the lack of follow-up in long-term complications. In the case of kidney transplantation, up to 60% of patients have tremor during their follow-up appointments7.
It is suggested that the mechanism of the neurotoxic effect is based on selected toxicity to glial cells and induction of oligodendrocyte apoptosis, but the clinical manifestation will depend on the time and severity of the exposure8.
Complications can be divided according to their severity: mild, moderate, and severe. Not only the most frequent manifestation that we can find is tremor, followed by headache, but also manifestations of both the central and peripheral nervous systems are frequently found (Table 1).
Table 1 More frequent neurological manifestations divided by severity
| Mild | Moderate | Severe |
|---|---|---|
| Tremor | Visual alterations | Coma/Altered consciousness |
| Headache | Cortical blindness | Confusion |
| Mood alterations | PRES | Psychosis |
| Neuralgia | Apraxia of speech | Seizures |
| Peripheral neuropathy | Leukoencephalopathy |
Less severe symptoms such as tremor or paresthesia can last months to years and revert spontaneously. Some of the more severe complications, such as visual blindness, are associated with an abrupt increase in tacrolimus levels. Multiple reports of paraplegia, symmetric polyneuropathy, dysarthria, and encephalopathy have been also reported in the literature.
Unlike kidney transplantation, a greater number of complications have been observed in liver transplantation, due to different mechanisms such as alteration in metabolism and circulating metabolites. The most frequent manifestations in these patients are alterations in the state of consciousness and seizures, and usually more severe manifestations9.
Sympathetic system
One of the most frequent complications of the use of tacrolimus is systemic arterial hypertension, but it is a consequence of a sympathetic dysfunction, possibly due to a modulation of NMDA and GABA receptors and modulating pre- and post-synaptic glutaminergic receptors10.
Clinical impact
In patients, a decrease in quality of life and an increase in morbidity and mortality have been observed, especially in liver transplant patients. In the first instance, severe complications can increase risk of death, but moderate complications can result in transplant rejection. Furthermore, systemic hypertension being a frequent complication results in cardiovascular and cerebrovascular risks. It is important to note that some of the complications will not be reversible11.
Image studies
The diagnostic standard is MRI, its indication focuses on moderate-to-severe complications such as in the case of seizures, altered state of consciousness, cortical blindness, and speech alterations. There are no prospective studies of the alterations by imaging, but a predilection for involvement of the white matter in the occipital region has been observed. In post-liver transplant patients, pontine and extra-pontine myelinolysis have also been identified (Fig. 1)12.
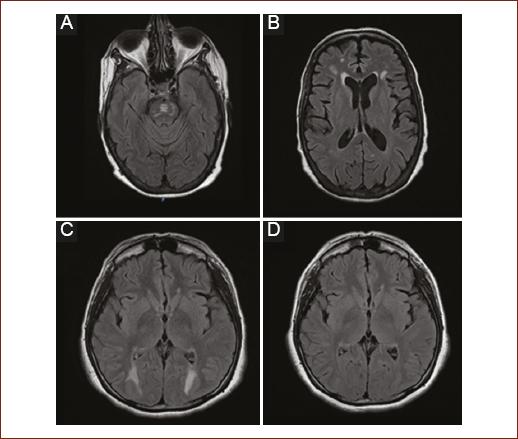
Figure 1 Most frequent radiological findings. A: T2 FLAIR sequence: hyperintense lesion in the pontine region corresponding to pontine myelinolysis. B: T2 FLAIR Sequence: multiple intra-axial hyperintense images located diffusely in white matter in relation to leukopathy. C: FLAIR T2 sequence: hyperintense intraxial images symmetrically in the occipital region. D: Control image 3 months later with reversibility of the lesions compatible with posterior reversible encephalopathy syndrome (PRES).
Management and treatment
The broad spectrum of manifestations should be treated first in a symptomatic way, and studies on serum levels should be carried out at the same time, also electrolytes should be measured, monitoring blood pressure, and evaluating imaging or neurophysiological studies are highly recommended.
If the serum levels are high and mild-to-moderate manifestations, the dose can be gradually decreased and the response assessed, while in severe symptoms the most appropriate is the suspension and adjustment of immunosuppression.
In the symptomatic treatment, the use of anticonvulsants, phenytoin, phenobarbital, and carbamazepine should be avoided since having the hepatic metabolism, serum levels might become erratic. Although empirically, the use of valproic acid and levetiracetam is recommended. Finally, it is recommended to control blood pressure levels and electrolyte alterations, mainly magnesium, which is a factor for the development of seizures13.
Conclusions
Tacrolimus is essential in the treatment of immunosuppression in the transplantation of solid organs, but its neurotoxic effects have a significant impact on the morbidity and mortality of patients. Patients with liver transplantation should be more vigilant because of the number of complications and severity.
In Mexico, as in the rest of the world, the rate of transplants is increasing, so these complications will become more frequent, and protocols for surveillance, prevention, and treatment must be developed.











 nueva página del texto (beta)
nueva página del texto (beta)


