Introduction
An important physical property of colloidal dispersions is the tendency of particles to aggregate and form clots that result in sedimentation of solids. The agglomeration processes and the resulting agglomerates are undesirable for most applications; Therefore, the synthesis and complex characterization of colloidal nanoparticles, based on their physicochemical properties, has been among the main research topics for the last years[1].
In the liquid medium the suspended particles are prone to meet and collide with each other, so colloidal stability is determined by these interactions between particles. Van der Waals attraction forces are primarily responsible for aggregation of the particles, which are long-range forces, so to improve colloidal stability it is necessary to counteract these forces, with equally long-range repulsive forces [2]. The main stabilization options are electrostatic (i.e. overlapping electrical double layers with similar charge) and polymeric. Polymeric and / or surfactants additives can influence stability through a variety of mechanisms [3].
The stability of colloidal particles depends on their electrokinetic properties. To ensure that the particles will be kept separate and avoid agglomeration and coagulation, colloidal particles should acquire similar primary charges and thus develop repulsive forces between them [4]. Primary electrical charges can be negative or positive. However, most of the colloids that exist in aqueous systems have a negative charge [5].
Proper choice and efficient adsorption of the surfactant used is one of the key problems to be solved in each particular case [3]. In the case of sterically stabilized nanoparticles in various liquid vehicles, the type and quality of the surfactants used will determine the efficiency of the surface coating of the particles and, consequently, the balance between attractive and repulsive interactions between the particles [6].
An important role is played by stabilizing agents protecting the nanoparticles formed from aggregation, making possible the preparation of particles with a diameter measuring no more than a few nanometers [7],[8],[9]. The most commonly used stabilizing agents are surfactants and polymers, with the help of which colloidal solutions containing nanoparticles are synthesized.
Water for the synthesis of the bifenthrin nanoparticles with the LASL technique is an ideal medium because it does not react with the material and it is innocuous for the final application. The use of different solvents can modify the insecticide effect of the nanoparticles and the evaluation could be wrong. Then, for the synthesis, an inert aqueous solution is used for the stabilization of the charges without modifying the insecticide properties of the nanoparticles.
For the purposes of the present work an electrosteric stabilization surfactant was used in the synthesis of bifenthrin nanoparticles. The stabilizer is a non-ionic polymer composed primarily of Triethylene Glycol Monododecyl Ether (S-4894), and the influence of this liquid medium on the stability as well as on the insecticidal activity of the colloids was evaluated.
Experimental details
We used a bifenthrin target, manufactured starting from a powder with a purity of 98%. 5 g of the powder were compacted with a hydraulic press and a force of 10 t during 1 h, using a one-inch diameter die. The bifenthrin target was fixed at the bottom of a 50 ml beaker, with 20 ml of a solution of a polymeric surfactant (S-4894) at 0.1 %. The height of the liquid above the target surface was kept constant at 12 mm during the process in order to keep the laser ablation efficiency constant.
The optical absorbance of laser-synthesized colloidal solutions was recorded in the wavelength range from 200 to 500 nm, using a UV-Vis spectrophotometer (Lambda 25, Perkin Elmer). The obtained spectra were significantly different from that of the nanoparticles synthesized in water due to the use of the surfactant.
Size, shape and crystal structure of the bifenthrin nanoparticles were analyzed by transmission electron microscopy (TEM) in a JEM-2010 equipment from JEOL, with an acceleration voltage of 200 kV. The specimen was prepared by placing a drop of the colloid directly on a carbon coated copper grid and allowing the liquid to evaporate at room temperature. The TEM images were processed by Image J software to obtain the size-distributions of bifenthrin nanoparticles.
The chemical composition of nanoparticles in the medium was determined with a gas chromatograph Agilent 6890 coupled with a mass selective detector Agilent 5973. The nanoparticles suspended in the liquid medium were measured directly by the equipment.
The absorbance spectrum of the surfactant used in the synthesis of nanoparticles at 0.1% dispersant solution in water exhibits a slight absorbance up to 200 nm, thus allowing the bands of the compound (bifenthrin) to be seen, and allowing the optical characterization of these samples by UV-Vis spectroscopy.
The targets were irradiated by a Nd:YAG laser that was operated at a wavelength of 532 nm, 10 Hz of repetition rate, a pulse duration of 5 ns, and an output laser energy of 70 mJ. The laser beam was directed to the target surface immersed in the liquid through a series of mirrors and a lens with 20 cm of focusing distance. The target in the beaker rotates at 15 rpm to avoid drilling. The samples of nanoparticles were synthesized using a solution of the surfactant at a 0.1% concentration. The synthesis was carried out during 15 minutes of ablation, using the lens 5 cm out of the focal distance closer to the target.
Results and discussion
Figure 1 shows the spectrum of the nanoparticles synthesized with surfactant solution at 0.1% as the liquid medium. It can be observed a band between 200 and 204 nm which appears in the absorbance spectrum of the colloid after the ablation procedure. As in reference [10], filtration after synthesis was used as an alternative procedure to improve the stability of nanoparticles and prevent agglomeration, since removing large particles that are detached from the target during synthesis avoids the attractive forces that they exert on the nanoparticles causing the agglomeration. Figure 1 shows the final unfiltered sample and the filtered one. As we can see there is a slight decrease in the intensity of absorbance after the filtering procedure.
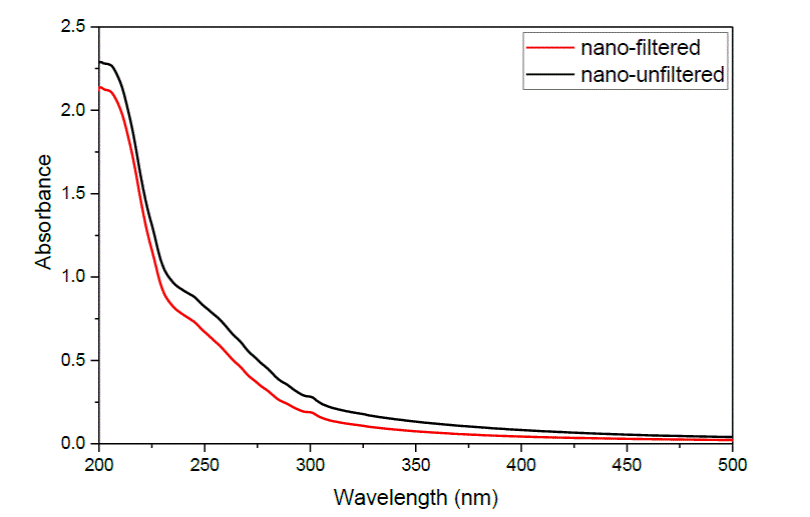
Figure 1 UV-Vis spectrum of the sample synthesized in a solution of S-4894 unfiltered (black) and filtered (red).
The colloids were monitored over time, and for this the absorbance spectrum was taken at different days after their synthesis. Figure 2 shows these measurements up to 4 weeks for unfiltered sample (Figure 2a) and after 13 weeks for the filtered sample (Figure 2b), after the synthesis of the colloid. For both samples the spectra keep their shape over time and only a slight decrease in intensity was observed. The fact that the spectrum does not change its shape over time could be correlated to a decrease of the agglomeration phenomenon of the nanoparticles.

Figure 2 UV-Vis spectra of the sample synthesized in the surfactant solution, a) unfiltered, and b) filtered.
To corroborate the presence of particles in the samples, it was dripped on a copper grid and analyzed in a transmission electron microscope. In Figures 3a unfiltered and 3b filtered sample, we can observe the presence of nanoparticles. In the unfiltered sample, the nanoparticles do not seem to be much dispersed, some particles supported in organic material were observed; however, it was not possible the identification of this organic material by this technique. In the case of the filtered sample in Figure 3b it can be seen the presence of homogeneously dispersed nanoparticles.
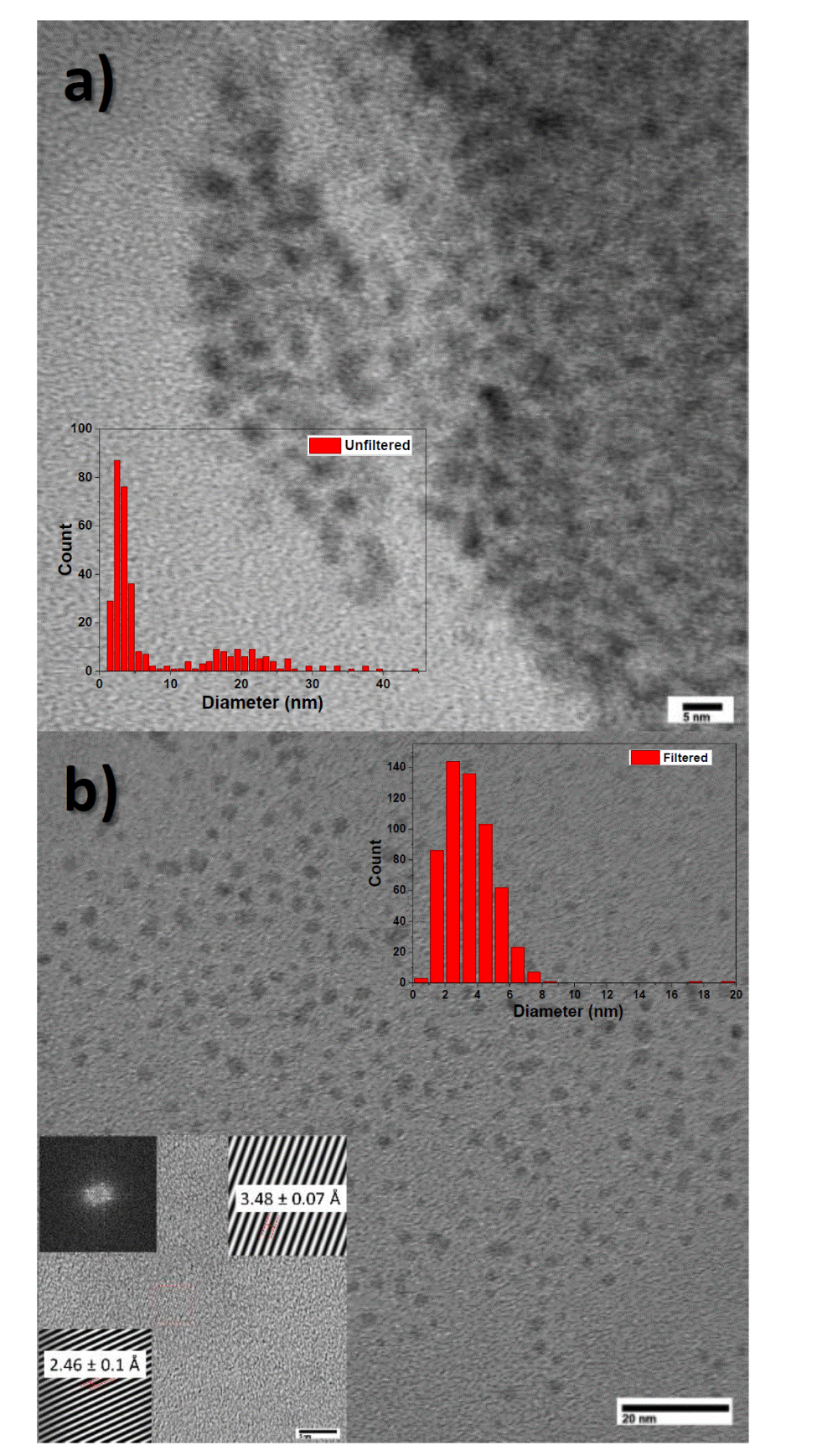
Figure 3 TEM micrographs of the sample synthesized in the surfactant solution a) unfiltered b) filtered. The HRTEM micrograph of the filtered sample is shown in the inset in b).
The size distribution of the nanoparticles was inserted in both Figures. In Figure 3a it can be observed a wide distribution with two groups of sizes. The particles most frequently found are those with diameter less than 10 nm. The particles with diameters ranging between 20 and 30 nm were removed after filtration as it can be observed in Figure 3b.
The second inset in Figure 3b shows high resolution micrographs where the inter-planar distances of some nanoparticles are indicated. The values 2.46 Å and 3.48 Å, correspond to the interplanar distances of the diffraction planes (404) and (520) of bifenthrin [10]. This result confirms that the structure of Bifenthrin is preserved in the nanoparticles.
In order to obtain the chemical composition of the observed nanoparticles, the sample was analyzed by mass-coupled gas chromatography. Figure 4 shows the gas chromatogram, where several bands corresponding to the surfactant are observed, as well as the bifenthrin band in minute 9.34, which indicates that nanoparticles of the starting compound bifenthrin are present in the sample.

Figure 4 Gas chromatograph of the sample synthesized in the surfactant solution. The inset shows the mass spectrum of the colloid.
The mass spectrum of the nanostructured sample, extracted from the gas chromatogram in minute 9.34, is presented in the inset of Figure 4, which shows the presence of the bifenthrin compound (at 181 m/z) confirming with this, that the use of the surfactant is not interfering with the nanoparticle chemistry as they retain the same composition of the bulk material.
Biological tests
To confirm that the surfactant is not interfering with the insecticide effectiveness of the nanoparticles, biological tests were performed with the drosophila melanogaster fly. The methodology used was the same as that described in reference [10]. Briefly, wild-type D. melanogaster flies (Canton-S) were raised on standard food. After that, one day old adults, males and females by separately were put in plastic vials in three groups of 25 each. Vials contained 0.8 g of culture medium (Formula 4-24 Carolina Biological Supply Company) with 2.5 ml of the bifenthrin colloid. Proper controls were included (surfactant solution 0.1%). For analyzing the insecticide effect, the number of death organisms was recorded in each vial every hour during the test. The count of the survivors was carried out to obtain the data of viability.
The biological tests were carried out using two colloids of nanoparticles. The sample called Nano A, corresponds to a fresh colloid, and sample called Nano B corresponds to the same colloid but after a week of fabrication. Figure 5 shows the results. The red line corresponds to the control group (surfactant solution, without nanoparticles) and it does not show insecticidal activity on flies. The samples with nanoparticles showed an important insecticidal activity reaching 50 % viability at the end of the first hour. And all the flies were death after a time between 5 and 7 hours. The difference between the colloids after 5 hours treatment is not statically important. This result also shows that the colloid after a week of fabrication has a good insecticidal activity.
Conclusions
The use of an electrosteric surfactant in the synthesis of organic nanoparticles by laser ablation of solids in liquids, markedly improved their stability, as well as allowed obtaining a higher concentration of nanoparticles in the medium.
The use of surfactant solutions as a liquid medium in the laser ablation of solids in liquids technique, under the synthesis conditions used, does not modify the chemical composition of the nanoparticles obtained
The surfactant is completely inert in the biological tests, so its use is compatible with insects without altering the insecticidal effects of the active ingredient.
The filtration process improved the stability of the nanoparticles since removing large particles from the colloid the agglomeration process decreases.











 nueva página del texto (beta)
nueva página del texto (beta)



