1. Introduction
Carbon Nanotubes (CNTs) are a promising material to be used on many innovative applications due to their physical, chemical and mechanical properties [1, 2]. Different researches involving CNTs incorporation to other materials suggest that better results are obtained when an homogenous dispersion occurs [3]. Generally CNTs functionalization enhances regular distribution into the material in order to facilitate the production of different electronic devices. The conventional methods to add functional groups to CNTs require aggressive treatments, using reflux techniques and highly concentrated acids, resulting in multi-step, expensive and non-environmental friendly processes [4-7].
Most general Multi-Walled Carbon Nanotubes (MWCNTs) acid treatments increment carbonyl and carboxyl functional groups concentrations. Hydroxyl groups are the most important on CNTs polymer nanocomposites applications. Blazej et al. propose a method to convert carbonyl and lactone groups into hydroxyl groups via sodium tetrahydroborate treatment [5]. Carbon nanotubes are employed also without functionalization like the solid-phase extraction materials for non-covalent compounds [8]. Li et al. reported air oxidation process using previously acid treated CNTs and a 480-750 ºC temperature range [9]. Dementev et al. described single wall carbon nanotubes oxidation using 400-770 ºC temperatures. [9, 10].
The aim of this research was multi-walled carbon nanotubes functionalization through thermal treatment at atmospheric exposure, using different temperature ranges. Further oxidized samples analysis was carried out to obtain morphological structure, element percent concentration and functional groups presence according to each temperature.
2. Experimental procedure
2.1 Materials and methods
MWCNTs were obtained by Chemical Vapor Deposition (CVD) into an experimental reactor previously developed and described by Gómez et al. [11]. Benzene and ferrocene were organic and organometallic precursors. Temperature was 760 °C with 70 ml/min argon flow.
CNTs samples were characterized and divided for subsequent thermal treatment. MWCNTs were set inside a quartz tube and placed into an electric furnace at 270, 300, 350 400, 500 and 600 °C temperatures for 30 minutes. The fully opened quartz tube, with 0.02 m diameter and 0.6 m length, was used in order to allow atmospheric oxygen exchange.
2.2 Characterization techniques
Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS), with a JEOL JSM-5910LV, analyzed MWCNTs morphology and elemental composition. CNTs infrared absorbance spectrum was registered through a Fourier Transformed Infrared (FTIR) Tensor 27 Bruker in potassium bromide pellets by 4000-400 cm-1 amplitude with 32 scans. Multiwalled carbon nanotubes average microstructure was studied by X-ray powder diffraction (XRD) in a Bruker D8 Advance diffractometer (Cu K _ = 0.1506 nm, 20 ≤ 2 ≤ 80 with 0.0355°/s). MicroRaman DXR Thermo Scientific (solid state laser 532 nm) was used for Raman spectroscopy.
3. Results
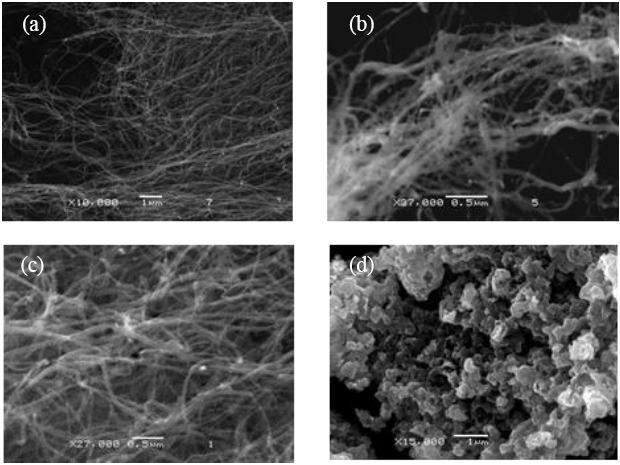
Oxidized MWCNTs samples below 400 °C, observed in Figure 1(b, c), resemble to pristine CNTs (Figure 1(a)). However carbon nanotubes were not observed in samples treated above 500 °C (Figure 1(d)). Different element atomic percentages were observed through EDS analysis for treated samples, as shown in TABLE I. Domratcheva et al. explained the Fe role in carbon self-organization into CNTs[12].
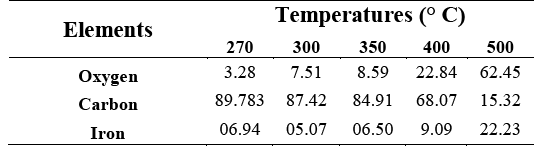
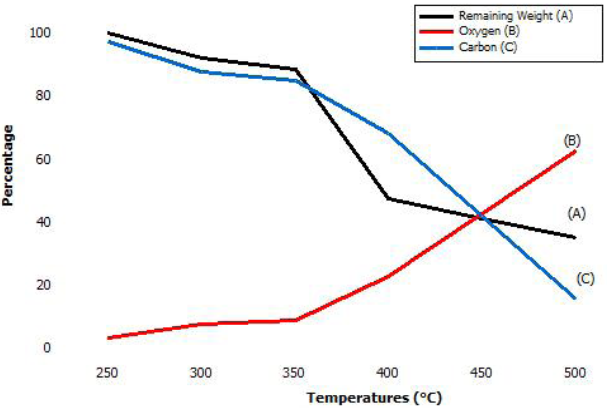
Atomic element percentage behaves differently according to the oxidation temperature Figure 2. Line A plots the remaining weight percentage. Line B shows oxygen atomic percentage increasing with temperature, starting from 2 to 63 %. Line C illustrated the 90-16 carbon atomic percentage decrease according to temperature increment.
A notable oxygen increment and a relevant sample weight loss started at 350 °C. Hence, carbon diminish significantly in contrast to other elements.
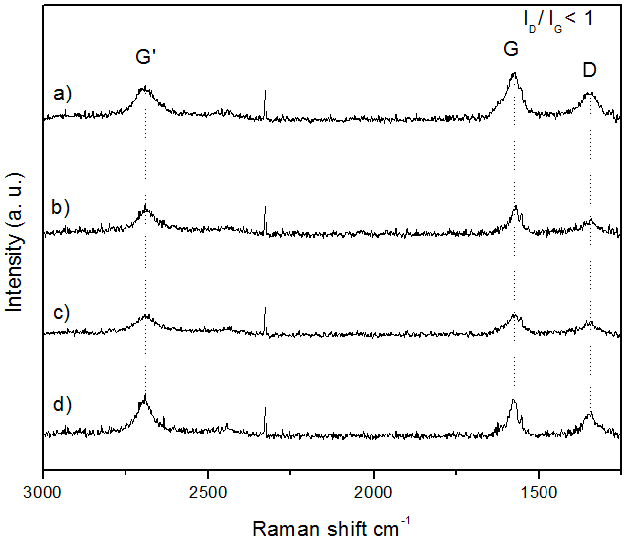
Figure 3 shows thermal treated carbon nanotubes Raman spectra. The G band (E2G Raman active modes) around 1575 cm-1, a typical band for graphite materials and for CNTs, was found in 270-400 ºC oxidized samples; thus corroborating carbon nanotubes presence [13].
The D band, characteristic of disorder in graphitic lattice or defects in nanotubes [14], was observed close to 1340 cm-1 in MWCNTs treated from 270 to 400 °C with different intensities. The spectra show another band at 2682 cm-1 attributed to the overtone of D band, called G' [15].
Oxidized well alligned carbon nanotubes were confirmed by Raman spectra considering ID/IG < 1 relation [16]. The highest relation for treated CNTs was 0.67 at 400 °C, suggesting major defects on this sample. Antunez et al. indicate that larger MWCNTs diameters showed a broaden D bandwidth measure by full width at half maximum (FWHM) [17]. The FWHM D bandwidth value decrease was observed through the treatment temperature increase; therefore it could be possible a MWCNTs diameter reduction.
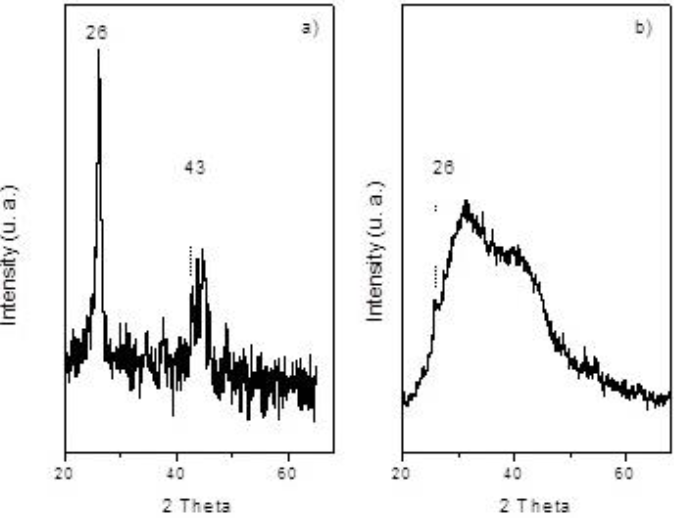
For pristine MWCNTs a reflection peak centered at 2θ ≈ 26° in XRD spectrum, is shown in Figure 4(a), which is a characteristic hexagonal graphite (002) reflection [18, 19]. Also this peak is noticed in all CNTs samples after thermal treatment, but could be overshadowed, "Fig 4b", due to defects in the crystalline structure or secondary products formed subsequently. Hassan et al. reported a broaden (002) peak on CNTs after an acid treatment [20]. The (100) peak with 2θ ≈ 43° was reported by Cao et. al [21].
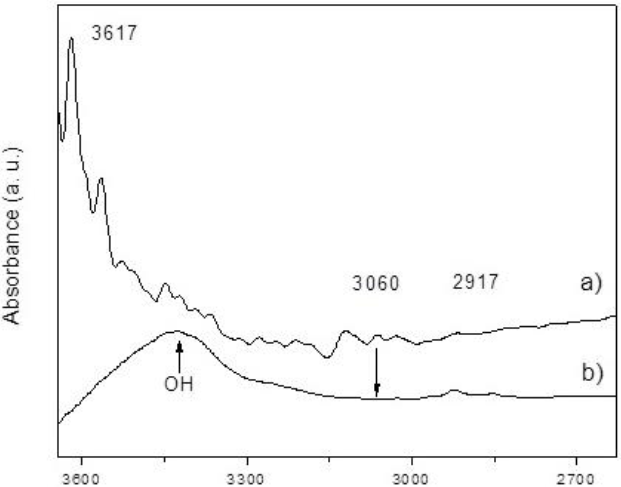
Pristine and oxidized carbon nanotubes FTIR spectra are shown in Figure 5 and Figure 6. The free OH stretch peak found in the MWCNTs at 3616 cm-1 widens and shifts to lower wave numbers, in thermal treated CNTs, indicating a hydrogen O-H bond. The CHx vibrations were found on region around 3060 to 2840 cm-1. The peak at 3060 cm-1 is associated to the stretch for sp2-CH in aromatic rings [22]. The oxidation of CNTs heated above 270° C could be proved by the disappearance of these signals. Intensities around 2917 cm-1 can be attributed to stretching vibration of CH2 and CH3; hydrogen interactions are common in carbon nanotubes obtained by CVD [23].
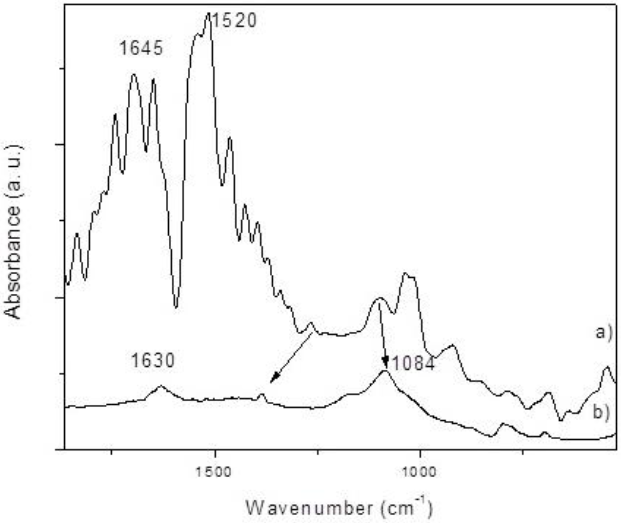
Lost in CHx species were reported by Douglas et al. after carbon exposure to ozone with 300 K temperature; leading to the production of -COOH species [24]. Bands at 1670-1600 cm−1 are related to the C=O conjugation with two aromatic rings. The stretching skeleton vibrations for most carbon materials, can be found in the 1582-1400 cm-1 range for pristine carbon nanotubes [23]. The 1630 cm-1 band for treated CNTs could be attributed to the conjugation of C=C with a carbonyl group, showing lower intensities. The 1084 cm-1 signal behavior might be explained by the shift of C-O absorptions to lower frequencies when the OH is attached to a ring [22].
4. Conclusion
This research proposes a methodology to functionalize carbon nanotubes avoiding traditional multi-step processes; thus leading to a simpler, faster and one step process. According to the temperature increments, there is a notable oxygen increase, however carbon content diminishes in samples; this is demonstrated by EDS and FTIR spectra. SEM micrographs did not show carbon nanotubes in samples treated above 500 °C. Results showed that carbonyl and hydroxyl functionalized groups could be obtained at 270-350 °C with minimum weight loss. The results shown by the proposed functionalization methodology could lead to a cheap and environmental friendly process to be further applied.











 nueva página del texto (beta)
nueva página del texto (beta)