1. Introduction
Green Nanotechnology encourages a vision of collaboration and development that uses scientific research to fundamentally move towards sustainability. In terms of design processes with less energy and resource expense, green methods applied to nanotechnology involve the improvement of performances and the use of materials without adverse consequences to human and environment [1].
The unique chemical and physical properties of Carbon Nanotubes (CNTs) have promoted an extensive research on nanostructural materials. There are several methods that have been used to produce CNTs. Chemical Vapor Deposition (CVD), which is one of the commonly used methods, offers low cost and flexibility [2]. The method is practical due to process parameters control, and the ease to scale up the synthesis process [3]. It has been reported that using the CVD method, organic precursors and metal catalysts, generate a good yield of CNTs with fewer defects [4-10]. The high cost of commonly used precursors have confined CNTs synthesis to research laboratories; therefore, the importance in searching for new natural renewable carbon sources that have low cost and are easy to obtain [11].
The emerging field of nanoscience applies green chemistry and green engineering principles to the synthesis of nanomaterials by achieving an understanding between nanotechnology, innovation and green chemistry [12, 13]. The analysis of traditional carbon nanotubes synthesis is encouraged to reduce environmental and health sideffects, incorporating alternative carbon sources in future methodologies designs. The present paper outlines an exploration on environmental friendly renewable precursors.
The purpose of this research was the synthesis of Multi-Walled Carbon Nanotubes (MWCNTs) throughout a sustainable process from butanol, diethyl ether, ethyl acetate and hexane by chemical vapor deposition with a stainless steel core as catalyst.
2. Experimental procedure
2.1 Materials and methods
CNTs were produced from butanol, diethyl ether, ethyl acetate and hexane with a stainless steel AISI 304 catalyst tube. A quartz tube of 0.0254 m diameter and 0.6 m length was used as reactor. The reactor was heated by an electric tubular furnace. Argon was used as carrier gas and to maintain an inert atmosphere during process. Precursors were loaded into the reactor. The temperatures process was 800 - 850 °C for butanol and hexane, 680 °C for diethyl ether, and 800 - 815 °C for ethyl acetate. Process was performed at atmospheric pressure. Carbon nanotubes growth required carbon source pyrolysis. CNTs time synthesis was between 30 to 40 min. The reactor cooled down at room temperature once the reaction was concluded. The nanotubes found on the catalyst surface were collected to further analysis.
2.2 Characterization techniques
CNTs morphology was analyzed by Scanning Electron Microscopy (SEM) with JEOL JSM-5910LV attached with an Energy Dispersive Spectroscopy (EDS) equipment in order to obtain element percentages. CNTs infrared spectra were registered through a Fourier Transformed Infrared (FTIR) Tensor 27 Bruker. CNTs average microstructure was studied from diagrams obtained by X-ray powder diffraction (XRD) in a Bruker D8 Advance diffractometer (Cu K _ = 0.1506 nm, 20 ≤ 2 ≤ 80 with 0.0355°/s). MicroRaman DXR Thermo Scientific (solid state laser 532 nm) was used for Raman spectroscopy.
3. Results obtained
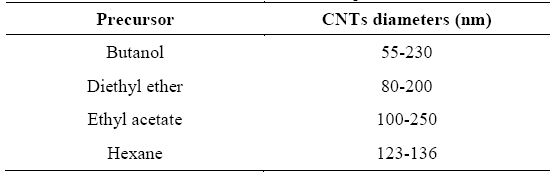
CNTs synthesized were depicted along SEM and EDS, both analysis provide a clear picture on the growth, overall structure and elemental composition of the nanostructures. These technics confirmed the presence of MWCNTs with some structural defects. Nanotubes diameters are summarized in Table 1.
Figure 1(a) shows CNTs from butanol with different shape and similar size. Samples contain carbon (99.16 %) and iron (0.84 %).
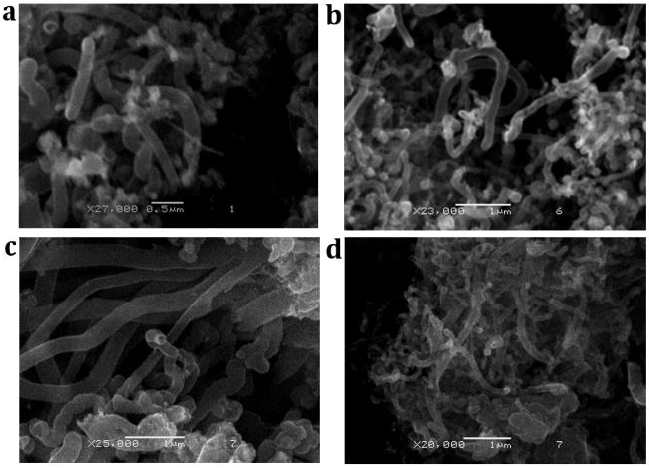
Figure 1 SEM micrographs of CNTs obtained from: (a) butanol, (b) diethyl ether, (c) ethyl acetate and (d) hexane.
SEM micrograph of CNTs obtained through diethyl ether is shown on Figure 1(b) with some nanoparticles attached to the nanotubes. EDS depicted carbon around 93% with a small presence of oxygen 5% and smaller percentages of iron, chrome and manganese.
The analysis of CNTs morphology synthesized with ethyl acetate (Figure 1(c)) indicated random growth. Carbon (94.77 %), oxygen (4.45 %) and iron (1.53 %) were found by energy dispersed spectroscopy.
Bulks of CNTs from hexane are observed in Figure 1(d). EDS was used to determine atomic percentages and results were 94.77 % of carbon, 4.35 % of oxygen, 0.57 % of iron and 0.35 % of chrome.
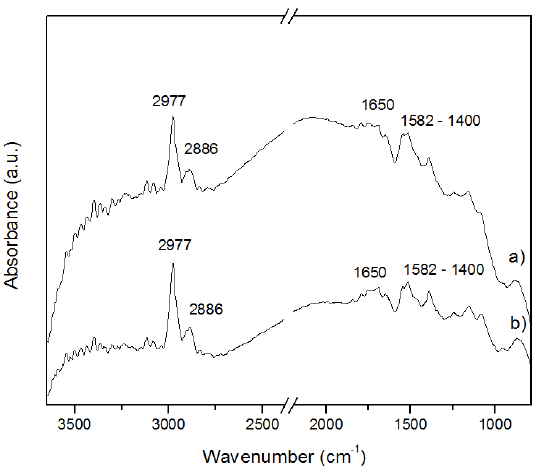
MWCNTs FTIR spectra are shown in Figure 2. The region around 3040 to 2840 cm-1 corresponds to CHx vibrations. The 3040 cm-1 signal fits in the stretch for sp2-CH characteristic of aromatic rings [14]. The signals from 2997 and 2886 cm-1 can be attributed to stretching vibration of CH2 and CH3 [15]; these vibrations are commonly found in multi-walled carbon nanotubes obtained by CVD [16]. The intensity at 1650 cm-1 is associated to C=O stretching of a conjugated ketone [15].
The carbon stretching skeleton vibrations for most carbon materials can be found in the range 1582-1400 cm-1, like the E1u from the sp2 hybridized carbon at 1570 cm-1 [15, 17]. The CNTs obtained from the four precursors showed similar signals and intensity peaks in FTIR spectra, however the intensities of carbon nanotubes from butanol and hexane were better defined.
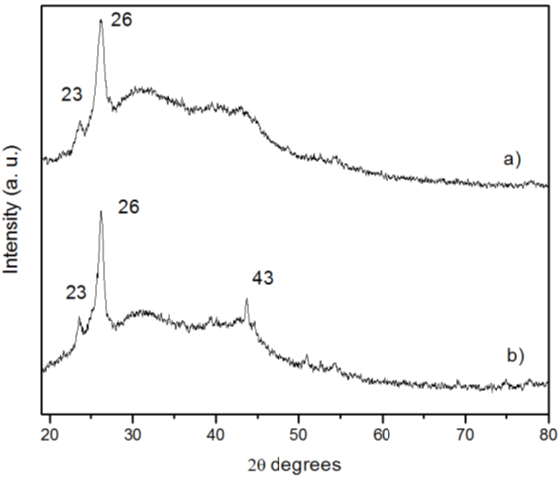
XRD was used for CNTs phase identification Figure 3. Spectra analysis showed a reflection peak centered at 2θ ≈ 23° related to graphene sheets loosely stacked, different from the crystalline graphite [18]. The located peak in samples with all precursors at 2θ ≈ 26° is characteristic to the reflection of hexagonal graphite (002) reported for carbon nanotubes [19, 20]. Figure 3(a) shows a peak at 2θ = 43° that correspond to (100) diffraction for CNTs [21].
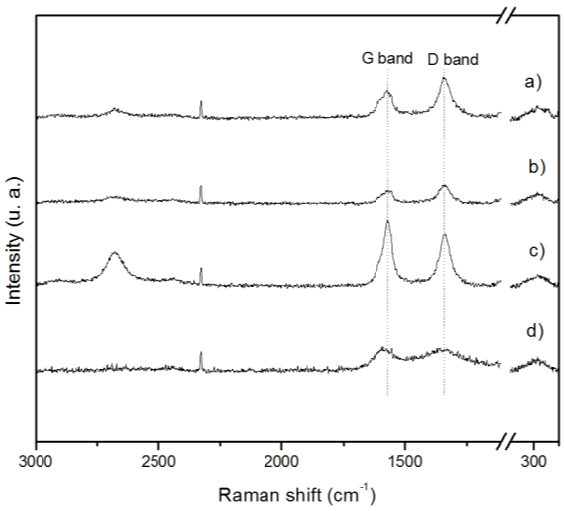
Raman spectroscopy was used to identify CNTs structures and defects that appear in the sample. The typical band for CNTs, called G band [22], was found at 1572, 1570, 1568 and 1589 cm-1 from CNTs produced with butanol, diethyl ether, ethyl acetate and hexane respectively in Figure 4. D band is assigned to defects in CNTs [23]. CNTs from hexane show a large width D band, similar to Shiratori et al. signal, indicating that the sample contains crystal defects and bond distortions in the CNTs [22]. Antunes et al., suggested that the ratio ID/IG is sensitive to structural defects but not diameter variation, also the line width of D band can be a parameter related to account overall defect density [23]; hence, the nanotubes produced by butanol and ethyl acetate contain similar imperfections (ID/IG = 1.4). A higher intensity of D band in these CNTs might be for the twister formation. The RBM Raman feature is usually weak to be observable in MWCNTs and the ensemble average of inner tubes diameter broadens the signal [24]. CNTs samples from all precursors show a significant broad with maximum intensities at 282 and 277 cm-1.
4. Conclusions
Multiwalled carbon nanotubes were synthetized by chemical vapor deposition from butanol, diethyl ether, ethyl acetate and hexane by chemical vapor deposition with a stainless steel core as catalyst. The presence of CNTs with minimum secondary products was confirmed, through SEM, EDS, FTIR, XRD and Raman. The experimental conditions, organic precursor and catalyst used in this research provide an alternative to synthetized carbon nanotubes reducing the environmental impact due to their low toxicity and relatively easy handling features.











 text new page (beta)
text new page (beta)