1. Introduction
Hexagonal molybdenum trioxide (h-MoO3) is an attractive material by its peculiar morphology when it is obtained as well faceted rods. This specific feature can be an advantage in terms of applications. J. Song et al. have studied the electrochemical properties of intercalation of lithium into the hexagonal phase of MoO3, showing that this material has potential application for secondary lithium ion batteries 1 2. According to another work by J. Song et al. the hexagonal phase presents photoluminescent properties with emission bands at 436, 606 and 668 nm when the excitation is at 330 nm 3.
Various synthesis methods have been reported to obtain h-MoO3 1 13. Almost all the methods to obtain h-MoO3 are based in hydrothermal treatments. S. Deki et al. have reported the h-MoO3 synthesis by liquid phase deposition. They showed that the diameter and length of the hexagonal rods can be modified changing the reaction time 12. For a 36 h of reaction time, they obtained rods with a diameter and length of 16 μm and 30 μm, respectively. Depending on the synthesis method, the hexagonal rods are defect free on the surface walls. A. Chithambararaj et al. report the effect of temperature of synthesis on the crystal structure of molybdenum trioxide using a hydrothermal method 13.
Characterization studies of the hexagonal phase have been reported in the literature. X-Ray diffractograms, Fourier transform infrared spectra (FTIR), Raman spectra 5 9 are known and present features clearly different compared to the other polymorphs of MoO3. After a synthesis process, these techniques let us to identify the presence of the h-MoO3 phase in a rapid way. Studies have also been performed by electron microscopy (SEM and TEM) for the h-MoO3 8 11; as well as XPS, magic-angle-spinning 1H- and 15N NMR spectroscopy studies 5 12. Each characterization technique gives information about the size, morphology, composition, crystalline structure of the rods.
The h-MoO3 phase has been subjected to studies of structural stability under thermal or pressure treatments. Thermal studies of the hexagonal phase of MoO3 have been reported by various research groups 2 5 8 10 11. Differential Thermal Analysis (DTA), has been used to determine the h-MoO3 to α-MoO3 phase transformation temperature. Another important aspect related to stability, has been studied recently by C. C. Zhang et al, they investigated the stability of the h-MoO3 phase under pressure, using a diamond anvil cell up to 28.7 GPa at room temperature 9. They found that a transformation from h-MoO3 to amorphous MoO3 is obtained at pressures starting at 5.6 GPa. Optical studies of the h-MoO3 phase have been done to determine the optical band gap of this material, according to the literature the optical band gap is in the order of 2.99 eV 4, 2.92-3.05 eV8.
The aim of this work is to present a novel method to prepare well-formed, defect free h-MoO3 rods. A detailed characterization of the as obtained material includes Scanning Electron Microscopy (SEM) and microRaman spectroscopy.
2. Experimental
2.1 Preparation
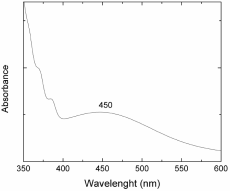
A molybdenum (sheet) piece of 0.1g was immersed into a previously prepared mixture of 60% HCL and 40% HNO3 (total volume utilized was 1 ml). After a given immersion time, the molybdenum piece was totally dissolved and the solution acquires orange-brown coloration. Molybdenum reacts with the mixture of acids to give water, nitric dioxide and complex ions MoCl4 ‾. Figure 1 shows the absorption spectrum of the as-obtained solution, it was obtained with a Cary 5000 UV-Vis-NIR spectrophotometer, in the range of 350-600nm. It can observe absorption bands centered at 370, 380 and 450 nm. This last band is related with the color of the solution (orange-brown). The bands at 370 and 380 nm are related to the absorption of nitric dioxide formed after the reaction process of nitric acid and molybdenum.
In order to obtain molybdenum oxide, the resultant solution was treated thermally in a furnace in air. To this purpose, a cylindrical glass (0.5 cm in diameter and 3.2 cm tall) was placed inside the furnace. The molybdenum solution was heated up to 100 oC for 45 minutes. At the end of this period of time 0.25 g of powder was obtained.
2. 2. Characterization
The as-obtained sample was characterized by microRaman spectroscopy to study the nature of the synthetized material. A micro-Raman system (LabRaman HR-800 of Jobin-Yvon-Horiba), which uses a He-Ne laser (λ=632.8 nm) as excitation source, was utilized in our study. A 100X microscope objective lens of an Olympus BX-41 optical microscope was used to focus the laser beam on the sample and to collect the scattered light. As per the morphological features of the obtained material, a scanning electron microscope (Jeol JSM6510 LV) was used to collect detailed images of the obtained structures. The as-obtained samples were set on graphite tape and the images were obtained through secondary electrons.
3. Results and discussion
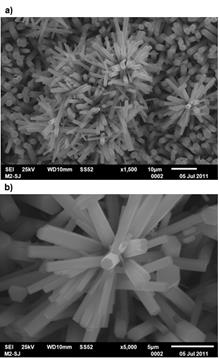
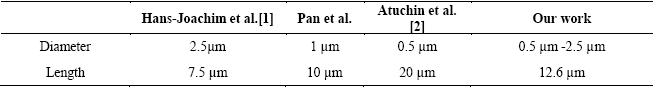
Figure 2 shows the SEM micrographs for the powder obtained when the solution was thermally treated at 100 oC for 45 min. A large amount of straight hexagonal rods are very well formed, as it can be seen in micrograph 2(a). It is worth noting that the typical lamellar form of α-MoO3 is not observed in the SEM images. The material formed in a homogeneous way, such that all the grown rods possess hexagonal geometry. Notice too how some rods are linked forming hierarchical structures. In micrograph 2(b) we can see the excellent faceted surface quality being very smooth in each hexagonal rod. In table 1 we compare our obtained results to those of the hexagonal rods obtained by other research groups. It is clear that micro or submicrosized rods in diameters can be obtained by the synthesis methods reported in the literature.
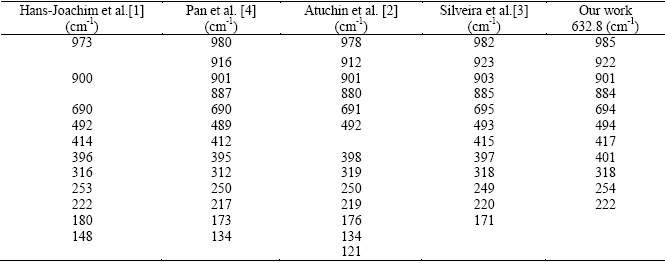
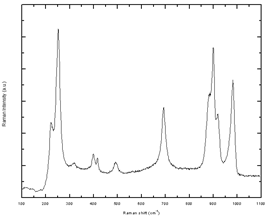
The Raman spectrum of the hexagonal MoO3 has been reported in the literature within the range 100-1000 cm-1. Table 2 summarizes the Raman frequencies for h-MoO3 reported by several research groups. Figure 3 shows the microRaman spectrum of the h-MoO3 rods that we obtained for the range 200-1100 cm-1. The Raman frequencies for h-MoO3 obtained in our work are in good agreement with those reported by Silveira et al. (see table 2). This result complements our SEM evidence and it confirms that the powder synthetized by this rather simple method, is constituted by hexagonal molybdenum oxide rods crystallized in the h-MoO3 phase. It is worth noting that the Raman frequencies of h-MoO3 depend on its composition.
4. Conclusions
The method of synthesis presented in this work is both simple and effective to prepare high quality hexagonal MoO3 rods. The method is capable of producing micro-sized defect free hexagonal rods. A more detailed study is required to determine the formation mechanism, out of the acid mixture and dissolved molybdenum, which that makes it possible to grow straight hexagonal rods. In view of other known synthesis methods to obtain h-MoO3, ours is considerable simpler.











 nova página do texto(beta)
nova página do texto(beta)