1. Introduction
In recent years there has been an increased effort to do research on the semiconducting properties of organic compounds due to some of their advantages over inorganic conventional semiconductors. The most relevant, which make them a desirable alternative for many practical applications, include their mechanical flexibility and ease of processing, lighter weight, lower cost, and above all the possibility of tuning their properties at will by changing the basic structure of the macro-cycle through chemical functionalization (a term known as "synthetic Tailorability") 1.
Organic semiconductors are beginning to be considered important functional materials because of the technological applications they have found in electroluminescent devices and in electronic devices such as transistors. Among them, phthalocyanine-based materials play a relevant role due to their interesting physical properties and particularly for the possibility of functionalization of the base structure with different chemical groups in order to improve their properties of interest.
In the fifties a lot of research on organic semiconductors was performed to understand their conduction properties. In the eighties it was visualized for the first time their possible use in electronic devices such as light emitting diodes and field-effect transistors 1 5. Among the organic semiconductors considered so far for these applications are pentacene, anthracene, organic polymers, phthalocyanines, etc.
In recent years there have been some strategies to make phthalocyanines useful for Langmuir-Blodgett (LB) film formation 1. Such an ordered assembly is desired, e.g., for the goal of observing well-defined charge transport properties in appropriate solid-state devices. In the case of vertical electron transport in a sandwich structure, it seems to be of advantage not to have a large distance between the phthalocyanine units in adjacent layers as might result from the presence of insulating long aliphatic chains.
Metal phthalocyanines (MPcs) are synthetic materials with interesting optical, electronic and structural characteristics that can be exploited in myriad applications. The semiconducting properties of phthalocyanines were observed for the first time in mononuclear (H2Pc and CuPc) phthalocyanine crystals obtained only by high vacuum deposition in 1948 2 3. The lack of solubility of most of the mononuclear phthalocyanine derivatives has always posed an impediment for exploiting their outstanding physical properties and therefore many research groups around the world have tried to synthesize other more soluble compounds, therefore to obtain a MPcs soluble with the modification of its compounds is very important. Within this family of functional compounds are also included metal phthalocyanines of lanthanide elements (radical phthalocyanines) which tend to form sandwich-type structures with higher solubility in common organic solvents than that of mononuclear phthalocyanine compounds. In addition, when the phthalocyanine units have substituents in the benzene sites, particularly long carbon-chain functional groups, where Erbium as metal and phthalocyanine compounds enhanced the solubility and others properties, due to the interaction of the central atom of Erbium and interaction between the molecule and Surface, the material obtained exhibits a very high solubility and can be deposited without difficulty as thin films using simple techniques such as spin coating 4 6. Where the solvent toluene is used and the solubility increased compared with the metal phthalocyanines. The above arguments explain that it is selected in the present work this type of substituent (Erbium)
On the other hand, the electrical conductivity of phthalocyanine materials can range between 10-15 and 102 S cm-1 and it is comparable with that of conjugated polymers 7 . The conductivity, of course, depends markedly on the solid-state structure and therefore the structural design features of the target organic compound have to be considered to favour the mobility of the charge carriers.
From all the above, our interest in developing octa-alkyl substituted radical phthalocyanine compounds of some lanthanide elements, as potential organic semiconductor materials, stems from one of their important properties: the inherent increased solubility that induces certain structural order when the films are deposited. The presence of several alkyl substituents in the Pc units of the bis-phthalocyanine compound is necessary to assure high solubility in common organic solvents as it has been shown in some previous works 8 9.
Therefore, in this study a sandwich-type octa-substituted Erbium (III) bis-phthalocyanine compound of increased solubility was synthesized. Then the pure product obtained from a chromatographic purification method was deposited as thin films by spin coating onto glass substrates to investigate its structural and optical properties. Interesting results were obtained with the different techniques of characterization.
2. Experimental Procedure
The target phthalocyanine compound Er(III)[Pc(3,6-C8H17)8]2 was obtained from a free octa-substituted phthalocyanine with octyl groups at the non-peripheral positions as follows: In a 100 ml round-bottom flask containing 48 ml of 1-octanol (previously dried and distilled) 35 mg of H2Pc(3,6-C8H17)8 are mixed with 131 mg of Erbium acetate (both reagents previously dried at 140 ° C under vacuum for 2 hrs.) under a nitrogen atmosphere. Then 0.190 ml of 1,8-Diazabicycloundec-DBU- is added (as a catalyst) with a micropipette. Heating is initiated until reaching the boiling point of solvent (180 °C), the reaction is left to proceed for 17 hrs under the same nitrogen atmosphere at reflux conditions. When the reaction is completed, the reaction mixture is allowed to cool to room temperature (RT). Once cooled the mixture is poured into 750 ml of methanol to precipitate the crude product which is recovered by filtration using filter paper. A transparent yellow filtrate and a dark green solid are finally obtained. To purify the target compound we proceeded to re-dissolve it in hexane to get a blue-green solution that was used to impregnate silica-gel. A chromatographic column was loaded for the purification of the product impregnated as indicated above. A mixture of tetrahydrofuran (THF)/acetone/acetonitrile/hexane in an 11:1:5:1 ratio was used as eluent. During elution a single green-blue band was observed and this was collected in 6 different fractions, the pure phthalocyanine compound obtained is a deep blue-green solid. The phthalocyanine material was then used to make thin films following the next procedure: First the glass substrates (Corning) were carefully cleaned using a standard cleaning process, which involves first degreasing xylene, then acetone and methanol, 10 min each in ultrasonic vibration, subsequently the sample was rinsed with deionized water, dipping in HF:H2O with a ratio of 7:1, rinsed in deionized water 10 . Then we proceeded to deposit the films using glass corning as substrates. The phthalocyanine films were deposited for two different concentrations (15 and 60 mg/ml) with a spin-coating system (Chemat Technology KW-4) at 2000 rpm for 30 s. The thickness films were measured using a Veeco Dektak 150 profilometer. X-ray diffraction analysis was carried out using a Bruker D8 discover, the incident radiation energy has a wavelength of 1.54 Å. Er(III)Pc2 films transmittance at RT conditions, was measured using an UV-Vis-NIR Cary 5000 system. The transmittance signal was collected from 400 to 800 nm with a resolution of 0.5 nm. PL response was measured at RT conditions using a Cary Eclipse fluorescence spectrophotometer with a pulsed xenon source whit a photomultiplier tube as a detector; spectrophotometer was controlled by means of a computer. The samples were excited using a wavelenght of 480 nm, and the PL response was recorded between 550 and 850 nm with a resolution of 1 nm. FTIR spectroscopy measurements were done using a Brucker system model vector 22. The surface morphology of Er(III)Pc2 films was also studied using a Scanning Probe Microscopy of Ambios technology Model Q-Scope 250/400 Nomad, operated in non-contact mode. A 4 × 4 μm2 scanned area was used for each topographic image, and a 460 µm-long single-crystal Si n-type cantilever operated at 12 kHz (type MikroMash SPM Probes) was used. Four different scans were done for each sample, showing good reproducibility. AFM images were analyzed using scanning probe image processor (SPIP) software 11.
3. Results
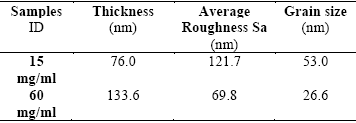
The thicknesses of the Er (III) Pc2 films were obtained by Profilometry, and the results are listed in Table 1. Figure 1 shows X-ray diffractograms for the Er (III) Pc2 films deposited on Corning glass with the two concentrations referred above. In this figure we can see for curve corresponding with concentration of 15 mg/ml one intense peak in 2ϴ = 7.46° and three incipient diffraction peaks at 9.24°, 9.46° and 11.80°; these last peaks could arise from noise, whereas for concentration of 60 mg/ml the main peak corresponds with 2ϴ = 6.84°, and additionally there is an almost vanish peak at 7.19°. The shift in main peak was associated with structural transformation from α-phase into β phase arise from temperature changes. Additionally to shift arise from temperature; the width of the peaks was affected by the presence of strain or stress in the mesh caused by defects, and compositional inhomogeneity. The peak broadening is proportional to the product of the deformation, which depends on the interplanar distance and changes in these, and the tangent of ϴ. The very broad peak centered at around 21° with small reflection angle shows the glass absorption 12 19. In Table 2 are listed the diffraction peaks of the Er (III) Pc2 films, and (hkl) values obtained of the literature to these phthalocyanine compound 20 25, which are similar to these obtained for Er (III) Pc2 films.
Figure 2 shows the transmittance spectra of the Er(III)Pc2 films; the spectra show some typical absorptions of the phthalocyanines in the region between 650 and 1800 cm-1. Table 3 lists the vibration modes found for these films. The UV-Vis spectra for the two different films are also shown in Figure 3; the peaks at wavelengths of 645 nm and 737 nm are assigned to the characteristic Q band of phthalocyanine materials which might be split into two bands due to the separation of the energy levels in the phthalocyanine ring dimer. The presence of two phthalocyanine rings of the bis-derivative in the solid state accounts for this. The peak at 737 nm is originated by an electronic transition from π to π *. Photoluminescence spectra of the Er(III)Pc2 films deposited at different concentrations are shown in Figure 4. Three major peaks are observed; a broad peak of medium intensity at 570 nm and beside its absorption of lower intensity at 591 nm. Finally the most intense peak with a narrow envelope is found at 606 nm. The peaks between 591 and 606 nm should correspond to the Q band and they are characteristic of the phthalocyanines 26 27.
To study the transitions between electronic states must take into account the quantum mechanical description of the complex, which implies the quantization of the vibrational levels. Within the Born-Oppenheimer approximation, the descriptions of wave functions derived of Hamiltonian, are products of electronic and vibrational wave. These functions are called vibronic and are of the form:
Vibrational progression of the absorption band (Figure 3) provides the energy for the excited electronic state vibration, while the obtained vibrational energy of the emission spectrum Figure 4 corresponds to the ground state. This explains why you see a difference in the energy levels of the Q band.
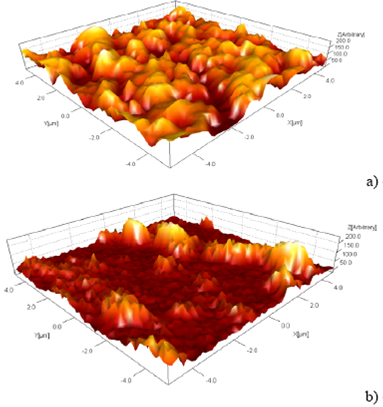
AFM images of the Er (III)Pc2 films are presented in Figure 5. All images exhibit a rough surface. It can be seen that the surface exhibits different characteristics depending on the concentration, which seems to influence the size of the grains (roughness), both their form and their composition. Average roughness increases with the decreasing of the concentration and these results are listed in Table 1.
4. Analysis and discussion of the results
Among the morphology studies of the deposited films of Er(III)Pc2 it is important to know their thicknesses, in this work they were obtained by the profilometry technique and the results are shown in Table 1. They show, as expected that the film made from the 60 mg/ml solution has a higher thickness, due to its higher concentration of phthalocyanine solution on the substrate compared to that of the sample with 15 mg/ml. The influence of the thickness of the films on their electrical properties made necessary to determine as accurate as possible the thicknesses of the two films prepared in this work. In the x-ray diffraction pattern of thin films deposited on a substrate, contribution from substrate to the diffraction can sometimes overshadow the structural characteristics from the thin film. In such cases, Glancing Angle X-ray Diffraction (GAXRD) is used to obtain the diffraction pattern of thin films. Considering this important fact and taking into account that our films deposited on Corning glass have very thin thicknesses, we performed the x-ray diffraction analysis using the GAXRD technique for two films with different concentrations whose obtained diffractograms are shown in Figure 1. In the latter we can only see a sharp peak at 2θ = 8.9° assigned to the phthalocyanine material and a very broad peak centered at around 21° with small reflection angle showing the glass absorption 11 13. The influence of the thickness and concentration of the films causes that some peaks are changed and also shifts to left both the sharp peaks and the broad ones and simultaneously reduces the intensity of the signal and makes curves with less fluctuations
It is well known that all MeIII(Pc+) 2 complexes show a strong marker band at 1310-1323 cm-1 which is been employed as a diagnostic for identify phthalocyanine p-radical anions Pc´ 14 19 28 31. In Figure 2 we depict the FTIR spectra of the films and analyze the first two bands, found from 1520 to 1570 cm-1 and from 1270 to 1300 cm-1, assigned to the diagnostic bands for the sandwich-type rare earth complexes, according to previous reports 31 34. The peaks found at 1355 and 1340 cm-1, which are associated to the radical anions 35 of the phthalocyanines, in agreement with some reports that assigned the 1341 cm-1 band to a stretching mode of pyrrole. These bands go from medium to strong intensity. In the range of 1300-1650 cm-1, it is found the isoindole coupling deformation where stretching bands are prominent.
Deformation vibrations in the plane C-H are assigned to the bands 1000 and 1305 cm-1, the bands at 1265, 1200 and 1160 cm-1 are due to C-H bending. The bands at 1115, 1070 and 870 cm-1 are assigned to isoindole breathing and coupling modes and to the deformation of the aza nitrogen respectively. The stretching band which corresponds to pyrrole vibration is found in 1341 cm-1, so the band 960 cm-1 is the pyrrole-N in plane bending, the band type rocking (wagging) at 802 cm-1 is attributed to the rare earths complex 36.
Even when the spectrum presented a phase shift between the two signals, it can be appreciated that the signals are relatively of the same magnitude, however the transmission of the sample with a concentration of 15 mg / ml is greater than that having a higher concentration, in the range of 1400 to 900 cm-1, and after of the wavenumber of 900 cm-1 the intensity is the same in both concentrations, indicating that the presence of a film with high concentration decreases the transmittance of the film. Lanthanide (III) bis-phthalocyanines with trivalent metal ions, showed the characteristic physical properties, however for their mononuclear analogues are unknown. Only the sandwich-type bis-phthalocyanine complexes form radical phthalocyanines of the general formula LnPc2 in which one of the rings is the well-known divalent Pc-2 and the other macro-cyclic ring is a radical anion Pc. The latter species has an unpaired electron which participates in an intramolecular charge transfer process between the two macrocycles which gives rise to two characteristic bands at 490 and 925 nm in the UV-Vis spectrum 37. In addition, the extent of such an electronic charge transfer has a direct effect in the band gap of these bis-phthalocyanine compounds and therefore it determines its electrical behavior as an intrinsic semiconductor. The electronic absorption spectra in the 400-800 nm range for the thin films of the new phthalocyanine material are shown in Figure 3 and exhibit all the expected features for similar bis(phthalocyaninato) lanthanide (III) complexes as described below. The Q band at 737 nm and its vibrational overtones at 645 nm for the films are broader compared to the same signals, appearing in a solution spectrum (see inset in Figure 3). The splitting of the Q band might be due to the separation of the energy levels in the phthalocyanine ring dimer. The presence of two phthalocyanine rings of the bis-derivative in the solid state accounts for this. This characteristic absorption of Pc materials in the blue-green region is originated by an electronic π→π* transition. There is also one weak and broad band at 475 nm. That accounts for the presence of a phthalocyanine π radical as expected in a lanthanide bis-phthalocyanine. The absorption features in the near UV region could not be observed for the films because the spectra were obtained only in the range of 400-800 nm 38 39. Figure 3, also shows that the film with a concentration of 15 mg/ml of Er (III) Pc2, has a higher percentage of transmittance than that of the film containing the 60 mg/ml solution. The film prepared with 15 mg/ml, being thinner, exhibited more transparency than the thicker 60 mg/ml one.
The transition to the Q band in Figure 4 for the photoluminescence spectra is attributed to the step (change) of the molecule from a normal excited state to an lower energy excited state, that produced a transition π → π * 39 42. The aforementioned transition is attributable to the emission of the higher excitation state in the LUMO level towards the HOMO level, and corresponds to a relevant absorption peak of the Q band. This energetic provision may explain the intensity that presents the peak at 606 nm. The luminescence of a higher energy state is more likely. In the case of the excitation of the LUMO level, the internal energy conversion is most likely, due to the small energy gap of separation between the HOMO and LUMO levels, i. e., due to the narrow band gap between the frontiers orbitals. By the presence of the lanthanide ion between phthalocyanine rings, the internal excitation can be provided between the energetic levels 40. The intensity of the luminescence spectrum in the type sandwich phthalocyanines is due to the number of electrons occupying the last orbital (4f orbital) of the metal ion (rare earth metal), so the 4f orbital is fuller the spectrum bands are more intense. Consequently, it can be seen that the intensity of the sample containing a concentration of 60 mg / ml is stronger than the one with15 mg / ml, this is due to the existence of a larger amount of Er(III)Pc2 presents in the deposited film. This corroborates the fact that the concentration of the dopant material on the surface influences the PL intensity of the film.
According with Table 1, the roughness and the grain size are larger for the samples of Er(III)Pc2 that have the lower concentration whose value is of 15 mg / ml, respectively. On the other hand, the sample of Er(III)Pc2 which has the higher concentration of 60 mg / ml its roughness and grain size are lower than the indicated previously with less concentration, having an average roughness of 69.77 and an average grain size of 26.59 nm, respectively. This noticeable fact that exhibits the Er(III)Pc2 with higher concentration may explain roughly as follows, the high concentration yields a greater thickness of the film consequently it permits the generation of islands of large sizes formed mainly in the volume of the film and not on its surface, such islands will only present part of their full dimensions; this fact reduces the density of them on the film surface and also the grain size. In contrast, films with less concentration and thicknesses, the formed islands are located on the film surface mainly, which brings as a consequence more roughness and grain size.
5. Conclusion
The synthesis of the Er(III)Pc2 with increased solubility described in this work allowed the facile standard characterization required for a new compound and the study of their structural and optical properties as a thin film. The results show that the films of Er(III)Pc2 with higher concentration exhibits a higher photoluminescence and lesser transmittance. In addition, the films produced from solutions with a higher concentration have exhibited a surface with less roughness and grain size. By virtue of the properties presented of the studied Er(III)Pc2 material it could be used for optoelectronic applications in building a photo-detector device and, perhaps in building an organic light-emitting diode.
The implementation of a reproducible protocol for the synthesis of the target compound of increased solubility which has permitted to make films by spin coating opens up the possibility for further studies in order to get a better insight on their intrinsic physical properties which permit us to understand correctly the optoelectronic properties of this family of compounds labeled as functional materials considered as novel ones with multiple promising applications.











 nueva página del texto (beta)
nueva página del texto (beta)