Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Superficies y vacío
versão impressa ISSN 1665-3521
Superf. vacío vol.27 no.1 Ciudad de México Mar. 2014
Plasma gradient modified scaffolds to generate a chemoattractant surface
Ramírez-Fernández O.*, Godínez R.*, Zúñiga-Aguilar E.*, López-Cobá C. A.**, Morales J.**, Olayo R.**
* Departamento de Ingeniería Eléctrica Universidad Autónoma Metropolitana, Unidad Iztapalapa Apdo. Postal 55-534, Iztapalapa, México, D.F. *odinramirezfernandez@gmail.com.
** Departamento de Física Universidad Autónoma Metropolitana, Unidad Iztapalapa Apdo. Postal 55-534, Iztapalapa, México, D.F.
Recibido: 28 de noviembre de 2013;
Aceptado: 27 de febrero de 2014.
Abstract
A gradient of plasma polymerization of pyrrole was generated to model a chemoattractant surface; it was obtained by changing the polymerization time to manipulate the thickness and the deposited layer. The polymerization was generated with a power of 30W on a cylindrical vacuum reactor, the coverslip was polymerized covered by a cavity with a surface exposed of 0.7 cm every 35 min., the cells seeded were liver stellate cells (Ito); it was evaluated against a coverslip with continuous plasma polymerization of pyrrole (Ppy). The results show similar protein secretions, but cells proliferation was selective in the surface gradient regions.
Keywords: Chemotaxis; Chemoattractant; Plasma Polypyrrole; Ito cells; Cell Culture; Modified Surfaces; Biomaterials.
1. Introduction
The aim of tissue engineering is to obtain tissue models that regenerate and replace a functional tissue or organ [1]. The tendency of the in vitro culture is to generate physiologically functional tissue to generate models of complex biological tissues [2].
The cell culture surfaces are designed to support cell growth and perform specific functions, as those tissues in vivo [3, 4]. The cell culture often requires designed substrates or scaffolds that allow control over cell proliferation and function [5].
At present, a set of biological, physical, and chemical methods are used to do surface modifications on biomedical devices and biomaterials [6]. These approaches can be used to tune a range of properties, including biostability and/or chemical inertness and cellular adhesion [7, 8]. The materials derived from the pyrrole are being used as an alternative to cultivate tissue. In biological environments the biocompatibility of the Ppy was tested both in vitro and in vivo [9, 10].
Plasma polymers differ from the conventionally synthesized polymers with their highly complex structure, with branched or cross linked chains [11]. The course of plasma polymerization involves the generation and dissociation of complicated reactive species such as electrons, ions, radicals, atoms and molecules. Therefore, only limited supporting literature detailed the relationships between the deposition kinetics with the physical-chemical properties of plasma polymers [12-16].
The final properties of the tissue will be in function of their movements responding to the chemical environment (chemotaxis) [17]. Gradient surfaces have recently been established as tools allowing the screening of cell proliferation towards its possible optimization. Plasma polymerization lends itself to surface gradient formation, since it supports the introduction of a wide range of surface chemistries and forms well-adherent layers on a range of substrates [18-21]. The technique also allows surfaces to be modified independently of the underlying substrate, with little change to surface topography [22].
In a modified culture surface it is possible to create a layer of cells with the most convenient geometry for the culture. In case of cocultures the different cells location plays a vital role because the organ functionality are strongly linked to the cell structure. For the particular case of the liver, the stellate cells synthesize extracellular matrix (ECM) components including collagen, which has the role of natural scaffolds then it is important to manipulate the regions where the stellate cells are growing.[10]
A chemoattractant model was created with the gradient coverslip using liver stellate cells. It was evaluated against a coverslip with continuous Ppy. The cells morphology were registered by microscopy, to corroborate the cell proliferation on the material, pictures of the samples with the cells were taken three times a week. Finally the metabolic functionality was measure with the protein secretion rate in the supernatant and was compared with the control material. In conclusion we obtain a Ppy surface that optimizes the conditions for the best cell culture.
2. Materials and Methods
2.1. Plasma Polymerization
The coverslips were modified in the surface with polypyrrole (Aldrich 98%) by plasma glow discharge with the following reactor conditions: pressure was on 9x10-2 Torr, measured with a vacuum sensor (Edwards Active Gauge Control), power of 30 watts, obtained from a source of radio frequency at 13.5 MHz (Dressler Cesar RF). Reaction time was 30 minutes [8]. The gradient of Ppy was generated with a power of 30W on a cylindrical reactor, and the substrate was moved into the cavity 0.7 cm every 35 min. as shown in figure 1.

2.2. Cell cultures
The stellate cells were routinely grown in a monolayer culture in the presence of a Williams E medium (Gibco 12551) supplemented with 10% fetal bovine serum (Gibco 16000), penicillin (100 units /ml) and streptomycin (100 mg/ml). Cells were grown at 37 °C in disposable plastic bottles (Nunc, USA), in a humidified atmosphere of 5% CO2 and 95% air. The medium was replaced twice a week, and cells were trypsinized and re-seeded every 7 days at a ratio of 1:3. [10].
3. Experimental Design
Stellate cells were seeded (5 x 105 cells per coverslip) directly on the gradient coverslip. It was evaluated against a coverslip with continuous Ppy surface polymerized at 30w for 20 min. Each coverslip was made in triplicate, n = 3, the dimensions of the coverslip are as follows: long 6.0 cm, width 2.0 cm; thickness 0.05 cm. The culture medium was sampled daily by 7 days. The experiment was repeated with the continuous coverslip.
3.1. Total protein rate
Bicinchoninic acid (BCA) protein analysis kit (Thermo Scientific 23225) was used to determine the total protein in supernatant, which works with colorimetry, at a wavelength of 562 nm. The standard curve was obtained initially obtained using Bovine Seric Albumin (BSA) at various concentrations provided by the kit. Samples of 0.5 ml were taken of the supernatant daily, they were placed in a 96-well plate to measure the concentration in a multimodal detector plates DTX 880 (Beckman Coulter) [10].
3.2. Distribution of living cells
To identify the cells those were staining with immunofluorescence technique of Hoetscht. This technique allowed observing the nucleus of functional stellate cells which were marked with fluorescent material. In addition to show the cell distribution in the modified coverslip; also it serves to identify the viable cells, since it reacts only with the functional cell nucleus. The photographs were taken in random way, in different zones of the coverslip to probe the different cells distribution.
3.3. Data analysis
Data are reported as mean ±SE for at least three independent experiments carried out in triplicate. The Origin package version 8.1 was used for statistical significance. Comparison among groups was done by means of ANOVA. Tukey's method was used for multiple comparisons. A P<0.05 was considered as statistically significant.
4. Results and discussion
The FT-IR spectrum for the control coverslip at 30 watts 30 minutes was presented in previous works, and the values show the vibration of primary amines at 3350cm-1, the aliphatic carbons at 2964cm-1, the nitrile groups at 2200cm-1, at 1624cm-1carbonyl groups [8]. Figure 2 shows the Ppy composition for the different sections of the gradient Ppy coverslip that change along the film thickness direction of the gradient. As show in the peak for the silicates (principal component of glass) at 850 cm-1 [23]; where the pointed blue line is for the clear glass and the yellow line is for the largest reaction time. The silicate peak absorbance, decrease in function of exposure time. The better culture regions show a largest aliphatic and primary amines peaks which are presents in the second, third and fourth increase exposure regions that correspondence to red, black and green lines. We suppose that those chemical groups increase the cellular adhesion and eventually the proliferation.
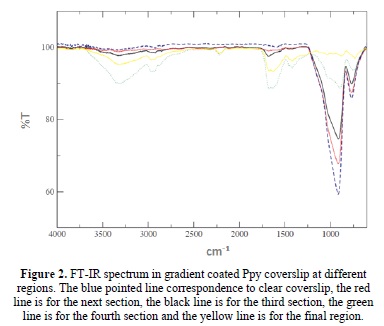
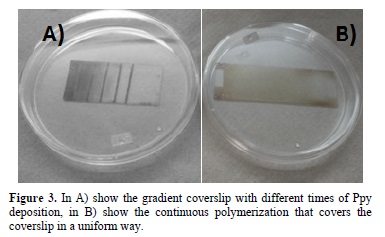
Figure 3 shows the results of the polymerization in the surface of the coverslips, been the principal indicator of the Ppy thin film, the brown color; this film cover all the structure in a way that depends of the time exposure. When the regions and the exposure time were controlled the coverslip shows a concentration gradient showed on the thickness of the Ppy.
In previous experiments the film thicknesses were characterized in base of the exposure time of the coverslips and they were correlated with the cell viability and their physiology, in base of these results we obtain a coverslip where we control the cell proliferation by regions with different Ppy concentrations. Because the stellate cell plays a scaffold role in the liver coculture and the extracellular matrix don't have a specific protein to measure, we decide to measure the total protein secreted to the supernatant.
The comparison of stellate cell cultures, using the coverslips with Ppy and the gradient coat was done, 5x105 cells by coverslip were used; passage 5; with 15 ml of culture medium for 5 days in a CO2 incubator.
Figure 4 shows, the cell culture on continuous coverslip (A) with a uniform cell distribution, and the cell culture on the gradient coverslip from the bigger to the lower Ppy deposition (B to F); the proliferation increases in the central zones, the typical cell shape in the scaffold is marked in a black circle.

To probe the cellular distribution in the gradient surface at day 5, the cells were marked with Hoetscht stain to identify the cell nucleus. Figure 5 shows the photographs of the stained cells with the fluorescent marker (A to F), it shows the cellular distribution on the gradient scaffold from less to greater concentration of Ppy, the dyed cells are shown in cyan color. G Shows the cellular distribution on the continuous Ppy coverslip, there are clusters of dyed cells in the entire surface.

In the gradient coverslip the cells prefer the central places to proliferate and it decrease in function of either lower or larger concentration of Ppy, since the F sample shows only 2 cells in the caption, the central regions have a similar concentration of Ppy as the continuous coverslip.
The cell adhesion, commonly known as; a direct reflection of the amount of Ppy functionalities on the surface of materials, did not correspond to the higher deposition time or to the thicker plasma polymerized thin film. We didn't observed morphological differences between the coated surfaces.
Figure 6 show the total protein rate in the supernatant of the cell culture medium. In the figure, the black squares line, represents the change of protein secretion in the medium after 5 days of culture on the gradient coverslip. The white squares line shows the total protein secretion in the continuous coverslip. As it can be seen, there is no significant difference in the two experiments.

Also, we analyzed the whole supernatant protein secreted, the results show similar values in the secreted protein when we used the Ppy gradient in comparison to the continuous surface, we obtained maximums of 14 μ g/ml with no significant differences. That suggests that the cell physiology is strongly linked to the cell distribution.
5. Conclusion
In this work is describing a novel method to fabricate plasma polymer gradients by using a tilted mask to control the concentration of plasma in the substrate. By forming a gradient layer of Ppy film, we created a chemical gradient surface suitable to examine the effect of cell proliferation and spreading on stellate cells protein secretion. The cells colony and morphology correlated with the continuous coverslip as show in the nucleus cell markers photographs. Cell protein response was similar in both experiments that show a comfortable surface for the cells. In conclusion we obtain a Ppy surface that models the conditions of chemoattractant surface to obtain a controlled cell culture.
Acknowledgements
The authors would like to thanks to Universidad Autonoma Metropolitana (UAM), Consejo Nacional de Ciencia y Tecnologia (CONACYT) (project 155239) and Instituto de Ciencia Y Tecnologia Del D.F. (ICyT-DF) (PIUTE 10-63276/2010) y (PICSA 11-14/2011), for their collaboration to bring to perform this work.
References
[1] R. I. Freshney, Culture of Animal Cells, a Manual of Basic Technique, Chapter 12, Subculture and Cell Lines 6th Ed. (Hoboken NJ, John Wiley & Sons, 2011) p. 187-206. [ Links ]
[2] M. Bokhari, R. Carnachan, N. Cameron and S. PrzyborskiJ. Anat. 211, 567 (2007). [ Links ]
[3] M Geetha., A.K. Singh, R. Asokamani, A.K. Gogia, Progress in Materials Science 54, 397 (2009) [ Links ]
[4] R.P. Lanza, R. Langer, J. Vacanti, Principles of tissue engineering. (Ed.Academic Press. 2000) p.215-289 [ Links ]
[5] S. R.Moosvi, R.M. Day, ActaBiomaterialia, 5, 76 (2009). [ Links ]
[6] Y.Shoufeng, L. Kah-Fai, D. Zhaohui, C. Chee-Kai, Tissue Engineering, 7, 679 (2001). [ Links ]
[7] G. Cruz, R. Mondragon-Lozano, A. Diaz-Ruiz, J. Manjarrez, R. Olayo, H. Salgado-Ceballos, M.G. Olayo, J. Morales Journal of Materials Science:Materials in Medicine. 23, 2583 (2012). [ Links ]
[8] Cruz G.J, Morales J., Olayo R. Thin solid Films 342, 119(1999). [ Links ]
[9] H. Biederman,,D. Slavinska, Surface and Coatings Technology 125, 371 (2000) [ Links ]
[10] O.Ramirez-Fernandez, R. Godinez, J. Morales, L. Gómez-Quiroz, M.C. Gutiérrez-Ruiz, E. Zuñiga-Aguilar, R. Olayo, Revista Mexicana de Ingenieria Biomedica 33, 2 (2012). [ Links ]
[11] Harding F. "Assessing embryonic stem cell response to surface chemistry using plasma polymer gradients" ActaBiomaterialia 8, 1739 (2012). [ Links ]
[12] I. Gancarz, G. Pozniak, M. Bryjak, W. Tylus, Eur. Polym. J. 38, 1937 (2002). [ Links ]
[13] G. Pozniak, I. Gancarz, M. Bryjak, W. Tylus, Desalination 146, 293 (2002). [ Links ]
[14] F. Truica-Marasescu, M.R. Wertheimer, Plasma Process. Polym. 5, 44 (2008). [ Links ]
[15] J. Xu, K.K. Gleason, Chem. Mater. 22, 1732 (2010). [ Links ]
[16] J.L. Baher, J. Yang, D.V. Kosynkin, M.J. Bronikowski, R.E. Smalley, J.M. Tour, J. Am.Chem. Soc. 123, 6536 (2001). [ Links ]
[17] D. Mangindaan, W. Kuo, C. Chang, S. Wang, H. Liu, M. Wang, Surface & Coatings Technology 206, 1299 (2011). [ Links ]
[18] L.-Q. Chu, W. Knoll, R. Förch, Langmuir 22, 5548 (2006). [ Links ]
[19] A.J. Choudhury, J. Chutia, H. Kakati, S.A. Barve, A.R. Pal, N.S. Sarma, D. Chowdhury,D.S. Patil, Vacuum 84, 1327 (2010). [ Links ]
[20] L. Denis, D. Thiry, D. Cossement, P. Gerbaux, F. Brusciotti, I. Van De Keere, V.Goossens, H. Terryn, M. Hecq, R. Snyders, Prog. Org. Coat. 70, 134 (2011). [ Links ]
[21] D. Mangindaan, W.-H. Kuo, Y.-L.Wang, M.-J. Wang, (Plasma Process. Polym. San Diago CA, pp. 7754 2010). [ Links ]
[22] A. Choukourov, H. Biederman, I. Kholodkov, D. Slavinska, M. Trchova, A. Hollander, J.Appl. Polym. Sci. 92, 979 (2004). [ Links ]
[23] Xia L., Xiupeng W., Dannong H. and Jianlin S., J. Mater. Chem., 18, 4103 (2008). [ Links ]














