Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Superficies y vacío
versión impresa ISSN 1665-3521
Superf. vacío vol.27 no.1 Ciudad de México mar. 2014
Obtaining and characterization of ZnSe nanoparticles from aqueous colloidal dispersions
Hernández R.*, Rosendo E.*, García G.*, Pacio M.*, Díaz T.*, Juárez H.*, Galeazzi R.*, Romano-Trujillo R.**, Nieto G.***
* PDS, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla 14 Sur y Av. San Claudio, edificio 103C, C.U. 72570 Puebla, Puebla. r2hdez@yahoo.com, enrique171204@gmail.com.
** Instituto de Energías Renovables, UNAM Privada Xochicalco S/N, 62580, Temixco, Morelos, México.
*** Facultad de Ciencias Químicas, Benemérita Universidad Autónoma de Puebla Av. San Claudio, C. U. 72570, Puebla, Puebla. México.
Recibido: 30 de septiembre de 2013;
Aceptado: 21 de enero de 2014.
Abstract
Structural, morphological and compositional characterizations of zinc selenide (ZnSe) nanoparticles (NPs) are presented. ZnSe NPs have been obtained by colloidal synthesis in aqueous solution using zinc nitrate (Zn(NO3)2) and elemental selenium (Se) as precursors, sodium borohydride (NaBH4) as reducing agent, a solution of sodium hydroxide (NaOH) and pentasodium tripolyphosphate (Na5P3O10) named Extran was used as surfactant. The pH was varied from 8 to 11 and the Zn:Se molar concentration was varied from 3:1 to 1:3. A cleaning process was applied to eliminate the by-products using hydrochloric acid (HCl). ZnSe NPs exhibit a cubic zinc-blende structure. The NPs size was estimated between 4.9 nm and 3.0 nm and depends on molar concentration.
Keywords: ZnSe; Nanoparticles; Colloidal method; Aqueous media.
1. Introduction
ZnSe is a wide band gap semiconductor material with bulk band gap of 2.7 eV at room temperature [1]. It emits and absorbs from the blue to the ultra violet range due to the quantum confinement effect that occur when the particle size becomes smaller than the exciton Bohr radius, so, these novel properties can be controlled by changing the particle size [2]. ZnSe NPs have attracted considerable interest over the years owing to their wide variety of applications, such as laser diodes [3], light emitting diodes [4], photodetectors in blue range [5] and solar cells [6]. ZnSe NPs have been synthesized by different methods, for example, sonochemical method [7], colloidal synthesis [8], organometallic synthesis [9], vapor phase synthesis [10], and thermal decomposition [11]. Colloidal synthesis is one of the most commonly used methods, it is based primarily on obtaining dispersed particles in a continuous medium, to carry out such synthesis, it is necessary to have precursors, solvent and a surfactant to allow the colloidal stability of the nanocrystal. Colloidal synthesis is frequently carried out in organic solutions, Murray et al [12] demonstrated the principles for this technique, it involves the decomposition of molecular precursors at relatively high temperature. Initially tri-n-octylphosphine oxide (TOPO) and tri-n-octylphosphine (TOP) were proposed as surfactants, which are very toxic and hazardous. Aqueous media is considered a friendly and safe alternative to carry out colloidal synthesis [13, 14], it allows obtaining NPs of good quality, this technique is easy and economical by not requiring the use of vacuum equipment during the synthesis or expensive control equipment. In this research work, we have synthesized and characterized ZnSe NPs obtained by colloidal synthesis in aqueous solution at low temperature, Extran was used as surfactant and hydrochloric acid (HCl) was used to eliminate the reaction by-products.
2. Experimental methods
For all the synthesis, Zn(NO3)2 (Meyer, 99.0%) and selenium powder (Aldrich, 99.5%) were used as precursors, NaBH4 (Sigma 95%) as reducing agent and Extran (MA 01) as surfactant, which also was useful to fix the pH of the solutions, HCl (Merck, 37%) was used to eliminate the reaction by-products by inducing a chemical precipitation. A typical synthesis was carried out by the following procedure. First Zn(NO3)2 was dissolved in deionized water at room temperature with constant stirring, and then Extran was added to the solution to protect zinc ions and modify the pH value. A second solution was prepared dissolving selenium powder and NaBH4 in deionized water under constant stirring for 13 minutes at temperature of 75 oC, so, selenium ions were obtained. Then the second solution was joined to the first, so, a single solution was obtained, which was mixed during 30 minutes at room temperature. A cleaning process was performed to remove the generated by-products, as first step HCl was added in the solution, it was stirred for 15 minutes, after that, the solution was left to stand for 15 minutes, allowing precipitation of particles, next the aqueous solution was extracted leaving the ZnSe NPs in the container bottom, As second step, deionized water was aggregated and the same procedure of agitation, precipitation and extraction was repeated, the two steps were carried out once again to ensure that the NPs were free of by-products. Finally NPs were dried at 45 oC for 2.5 hours. In these experiments, the molar concentrations were varied from 3:1 to 1:3 and the pH of the solutions was varied from 8 to 11. Measurements of XRD were made using a Bruker AXS D8 discover diffractometer at room temperature with monochromatic Cu Kα radiation (λ = 1.5428 Ǻ). The XRD results were obtained in a range of 20° to 80° with intervals of 0.02° and step time of 1 s. Also SEM and EDAX analyses were made to study the morphology and composition respectively, these analyses were carried out with a Hitachi SU-1510 Scanning Electron Microscope.
3. Results and discussion
During the process ZnSe NPs were obtained, however, unwanted by-products were also generated from the reactions during the synthesis. The proposed chemical reactions are described as follows, when zinc nitrate is dissolved in water, zinc ions and nitrate ions are obtained, when surfactant Extran is aggregated, it prevents that zinc ions react with the medium and form stable compounds, as it is shown in Reaction 1. Additionally, Extran can modify the pH of the solution because it contains NaOH.

In reaction 2, sodium borohydride is used as reducing agent to obtain selenium ions, the reaction is carried out at 75 oC due to selenium is insoluble in water at room temperature.

For reaction 3, the products of reactions 1 and 2 are mixed to obtain ZnSe NPs, in this part, the surfactant is again formed with some unwanted by-products.
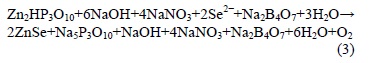
Reaction 4 shows the case when HCl is added. Then, colloidal particles are separated from the surfactant, giving rise to the formation of sodium chloride (NaCl). Then simultaneously ZnSe NPs are precipitated to the bottom of the container while unwanted by-products remain soluble in water. Therefore when the solution is extracted, byproducts are also removed.

Structural characterization of ZnSe NPs was carried out for all samples. Figure 1a) shows XRD patterns obtained from a sample prepared at pH 8 and molar concentration ZnSe, 1:1. This figure shows localized peaks at 2θ = 27.2o, 45.2o, 53.5o and 72.6o, which are related to ZnSe, according to the ICDD card number 01-071-5977, corresponding to the crystallographic planes (111), (220), (311) and (331), further there are some peaks of byproducts at 2θ = 23.5o and 29.7o related to selenium, which correspond to the planes (100) and (101) of the hexagonal phase, which are adjusted to the ICDD card number 00-006-0362, also two peaks appear at 2θ = 29.36 o and 38.9 o corresponding to NaNO3, which are adjusted to the ICDD card number 00036-1474, peaks localized at 41.16o and 43.55 o are related to Na2B4O7 in agree with the ICDD card number 00-0291179. Figure 1b) corresponds to sample after cleaning process, where the sample is free of by-products, it is possible to observe, how the cleaning process removes byproducts generated in colloidal synthesis.

Two sets of samples were obtained to observe the effects on the particle sizes due to the variation of some synthesis parameters. For the first group of samples, the pH value was varied from 8 to 11 with a molar concentration 1:1 corresponding to ZnSe. Figure 2a) shows the patterns for samples obtained at different pH values. For the second group, molar concentration was varied as follows 1:3, 1:2, 1:1, 2:1 and 3:1, for these cases the pH was fixed at 8. Figure 2b) shows the change of XRD patterns for samples obtained varying the molar concentration. In these set of XRD patterns, peaks related to selenium appear for samples with molar concentration 1:3 and 1:2, this is because the selenium concentration exceeds that of zinc and a portion of selenium is not reach to combines with zinc to form ZnSe.

The crystal size was estimated from the XRD patterns using the Debye-Scherrer equation [15] and taking the full width at half maximum (FWHM) of peaks located at 29 = 27.2o, 45.2o and 53.5o. Figure 3a) shows the relation between the particle size and the pH. The sizes calculated for this group of ZnSe NPs were between 3.9 nm and 4.5 nm. Figure 3b) shows the relation of particle size and the molar concentration, the sizes calculated for this group of ZnSe NPs were between 3.0 nm and 4.9 nm. As it is possible to observe, when the pH value of the solution is increased, the particle size does not change significantly and the corresponding peaks to ZnSe exhibit FHWM similar. But when the zinc concentration is increased the FHWM of the corresponding peaks to ZnSe increases, therefore the particle size decreases.

Morphological characterization of NPs ZnSe was carried out by SEM, a set of three representative SEM images are shown as follow. Figure 4a) shows morphology for sample with molar concentration 1:1 and pH 8, where appears agglomerates with semi-spherical and rod shapes, but when pH is increased to 10 with the same molar concentration (see figure 4b)), rod shapes are increased, this is because to raise the pH value, the Extran amount is increased, which implies, that surfactant influences the growth of rod-shapes, however, when zinc concentration is increased to 3:1 and pH remains at 8, the agglomerates size and agglomerates concentration were decreased, (see figure 4c)), additionally some wire shapes appear.

Compositional analysis of the ZnSe NPs was done by EDAX. Figure 5 shows a typical spectrum of ZnSe Nps, where it is possible to observe only presence of zinc and selenium, which is in agreement with the results obtained with x rays analysis.

4. Conclusions
ZnSe NPs were obtained using the method of colloidal synthesis in aqueous media, Extran was used as surfactant, the ZnSe NPs shown a good structural quality. The generated by-products were successfully removed using a cleaning process with HCl. The XRD studies showed that the ZnSe NPs exhibit a cubic zinc-blende phase structure. The size of ZnSe NPs was estimated between 3.0 nm and 4.9 nm. It was determined that the molar concentration variation influences the NPs size, while variation of pH value does not influence significantly the NPs size. The SEM analysis showed that the morphology of the NPs can change if we modify either, the pH or molar concentration, also it was confirmed the NPs composition by EDAX analysis.
Acknowledgements
The authors wish to thank the VIEP-BUAP by project funding provided by ROAE-EXC12-I, Likewise, Rogelio Hernández Hernández thanks to CONACyT for the PhD scholarship number 331572, and also to project CONACyT-UNAM 123122 (LIFYCS) for using SEM/EDS Hitachi SU1510.
References
[1] A. Sadao, T. Tsunemasa, Physical Review B 43 9569 (1991). [ Links ]
[2] Y. H. Chang, M. H. Chieng, C.C. Tsai, M. C. Harris Liao, F. Chen, Journal of Electronic Materials, 29, 173 (2000). [ Links ]
[3] C. H. Zhang, P. B. Meng, B. Q. Yao, G. Li, Y. L. Ju, Y. Z. Wang, Laser Physics, 21, 44 (2011). [ Links ]
[4] M. A. Hines, P. G. Sionnest, Nanocrystals, J Phys Chem B, 102, 3655 (1998). [ Links ]
[5] K. Lin, S. J. Chang, Y. K. Su, Y. Z. Chiou, C. K. Wang, IEEE Trans Electron Devices, 52, 121 (2005). [ Links ]
[6] D. H. Kim, Y. H. Lee, D. U. Lee, T. W. Kim, S. Kim, S. W. Kim, Opt Express 20, 10476 (2012). [ Links ]
[7] J. Zhu, Y. Koltypin, A. Gedanken, Chem. Mater.12, 73 (2000). [ Links ]
[8] L. Li, N. Pradhan, Y. Wang, X. Peng Nano Lett., 4, 2261 (2004). [ Links ]
[9] D. J. Norris, N. Yao, F. T. Charnock, T. A. Kennedy, Nano Lett. 1, 3 (2001). [ Links ]
[10] B. Xiang, H. Z. Zhang, G. H. Li, F. H. Yang, F. H. Su, R. M. Wang, J. Xu, G. W. Lu, X. C. Sun, Q. Zhao, D. P. Yu, Appl. Phys. Lett., 82, 3330 (2003). [ Links ]
[11] J. W. Lee, S. M. Lee, Y. D. Huh, C. S. Hwang, Bull. Korean Chem. Soc., 7, 31 (2010). [ Links ]
[12] C. B. Murray, S. Sun, W. Gaschler, H. Doyle, T. A. Betley, C. R. Kagan, IBM J. Res. Dev. 45, 47 (2001). [ Links ]
[13] J. Chomoucka, J. Drbohlavova, P. Businova, M. Ryvolova, V. Adam, R. Kizek, J. Hubalek, Electronics Technology, 2009. ISSE 2009. 32nd International Spring Seminar on Electronics Technology (ISSE). [ Links ]
[14] R. Romano-Trujillo, E. Rosendo, M. Ortega, A. M. Sanchez, J. M. Gracia, T. Diaz, G. Nieto, G. Garcia, J. A. L. Lopez, M. Pacio, Nanotechnology 23, 185602 (2012). [ Links ]
[15] I. C. Baek, S. I. Seok, N. C. Pramanik, S. Jana, M. A. Lim, B. Y. Ahn, C. J. Lee, Y. J. Jeong Colloid Interface, Sci. June, 310, 163 (2007). [ Links ]














