Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Superficies y vacío
versão impressa ISSN 1665-3521
Superf. vacío vol.25 no.3 Ciudad de México Set. 2012
Plasma copolymerization of pyrrole and ethylenglycol to obtain porous polymers
González-Torres M.1, Cruz G. J.*, Olayo M. G., Gómez L. M.1, López O. G.
Departamento de Física, Instituto Nacional de Investigaciones Nucleares Carr. México-Toluca, km 36.5, Ocoyoacac, Edo. Mex., CP 52750, México. Correspondencia: guillermo.cruz@inin.gob.mx
Sánchez-Mendieta V.
1Posgrado en Ciencia de Materiales, Universidad Autónoma del Estado de México, Facultad de Química Paseo Tollocan esq. Paseo Colón, Toluca, Estado de México. C.P. 52000.
De Jesús C.
Instituto Tecnológico de Toluca Av. Tecnológico s/n, Col. La Virgen, Metepec, Edo. Mex., CP 52140, México.
Recibido: 17 de diciembre de 2011
Aceptado: 12 de abril de 2012
Abstract
A study about processing and synthesis by plasma of porous biomaterials based in pyrrole and ethylene glycol is presented. The objective is to obtain porous copolymers with NH and OH groups to be applied as biomaterials. The monomers were copolymerized by resistive glow discharges in a tubular glass reactor at 10-1 mbar and 40-60 W using iodine as dopant and water as oxidant agents. The polymers were obtained as powder and thin films with some pores. The films were swollen with water and subjected to a treatment based on liquid absorption and rapid temperature changes to freeze the liquid trapped in the copolymer. With this procedure new pores were created, some isolated and others interconnected with average diameter between 0.13 µm and 10.75 µm. The morphology was studied by SEM. The results indicate that this material could increase the interaction with cells with the enlarged surface provided by the pores.
Keywords: Pores; Plasma; Polymers; Lyophilization; Ethylenglycol; Pyrrole.
1. Introduction
Porous materials have voids, cavities or channels, closed or interconnected with or without communication with the external surface. Pores can form complex materials because they open the possibility to introduce other chemical agents inside with application in many fields. In medicine they can be used in orthopedic implants or in the release of drugs previously collocated inside the pores [1, 2].
Depending on the size and configuration of pores, implants with these materials have the possibility to influence the growing of cells through the interconnected trajectory of pores or through the interaction of surfaces with open voids in contact with fluids and cells [3,4]. Most human cells are between 1-10 µm in size; therefore biomaterials designed to interact with these cells should be compatible with such dimension, although the size and morphology of pores has influence depending on the specific activity of cells [5-8].
Considering these characteristics, this work has the objective to develop porous copolymers of polypyrrole (PPy) and polyethylenglycol (PEG) by plasma. The non-porous version of these copolymers has shown compatibility with the central nervous system, among other causes, due to the participation of NH and OH groups and because they do not use in their synthesis other chemical reagents except monomers and dopants. The pores inside the PPy-PEG/I matrix of this work should be close to the size of these human cells.
2. Experimental section
The copolymers were synthesized with resistive electrical glow discharges in a tubular glass reactor 9 cm diameter and 26 cm length. The reactor has a stainless steel flange in each side with 3 access ports. One electrode can be inserted in each flange with 7 cm diameter. The electrodes were connected to an Advanced Energy RFX 600 power supply. The synthesis conditions were: 10-1 mbar, 13.56 MHz, 40-60 W during 180 min.
The monomers, ethyleneglycol (Tecsiquim, 99.5%) and pyrrole (Aldrich 98%), the dopant, Iodine (Aldrich 99.8%) and the oxidant, distilled H2O, were placed in separated containers and connected to the reactor ports. The ethylenglycol was maintained at 50°C. All reagents mixed inside the reactor during the synthesis.
Iodine atoms were inserted in the structure with the purpose to create sites with different availability of electrons than the groups of the monomers. This could help in the transport of electric charges, because one possible application of these copolymers is in the central nervous system which transfer information through ionic pulses, so the material should not obstruct these signals.
The function of water was to provide ions formed with the electric field that once accelerated collide with the molecules of pyrrole and ethylenglycol.
With this action, the monomers excite, form radicals and combine chemically to form random copolymers of both monomers. After the synthesis, the polymers formed on the reactor walls were swelled with distilled water and removed with a thin spatula. The thickness of the films was measured with a Mitutoyo micrometer. The structure was studied with a Magna 550 Nicolet infrared spectrophotometer. The morphology was studied with a Jeol JSM-5900LV scanning electron microscope and the micrographs were processed with the Olympus Measure IT program.
3. Results and discussion
3.1 Thickness of films
The polymers grew as stacked films with an average total thickness of 23.6 µm for the PPy-PEG-I synthesized at 40 W and 69 µm for the one at 60 W. Plasma polymers usually grow as consecutive layers due to small instabilities in the synthesis conditions. Some films were separated in individual layers to study their porosity and morphology.
The average thickness of the individual copolymer films was 5.2 µm and 15.6 µm for the samples synthesized at 40 W and 60 W, respectively. The ratio between the average individual and the total thickness of the films was 4.54 for 40 W and 4.4 for 60 W. These results indicate that the increase of power directly affects the total thickness of the copolymer; however the individual layers tend to maintain a ratio of approximately 4.5.
3.2 Morphology
PPy-PEG-I copolymer films have different characteristics on the surface, from smooth segments to increasing porosity and roughness with the energy applied to the synthesis, see Figure 1. These characteristics could be due to the difference in reactivity of each monomer which produces uneven morphology. Thus, when the power increases, the reaction rate rises, increasing the uneven growth of polymers.
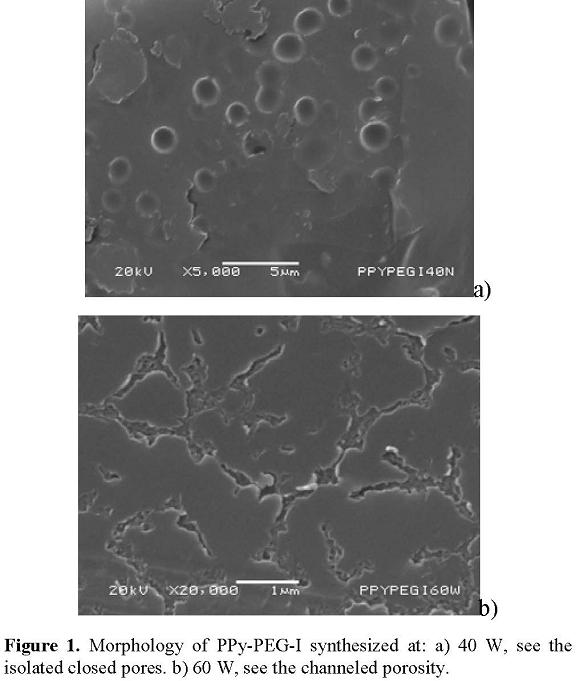
Porosity may appear as channels or voids on the surface. Figure 1(a) shows a surface of PPy-PEG-I synthesized at 40 W with isolated pores with small depth and circular form. The diameters go from 120 nm to 1790 nm with harmonic mean at 992 nm. The copolymer synthesized at 60 W is basically formed by interconnected channels with different width and depth; see Figure 1(b).
Pores can also be induced in some material by applying different steps of sequential treatments of liquid absorption and freezing with rapid changes of temperature. The most common liquid used in the first step is water, although other solvents for specific treatments can be used. The liquids have the function of penetrating the material to later freeze them creating spaces to host the solid inside. When the solid melts and evaporates, the voids remain in the material. This process is known generically as lyophilization.
To promote the porosity in the copolymers of this work, the films were immersed in distilled water for 3 min and later submerged in liquid Nitrogen during approximately 15 min. After that, the films were left in the atmosphere to dry and reach ambient temperature. This process was repeated with different polymeric samples using ethanol or acetone instead of water.
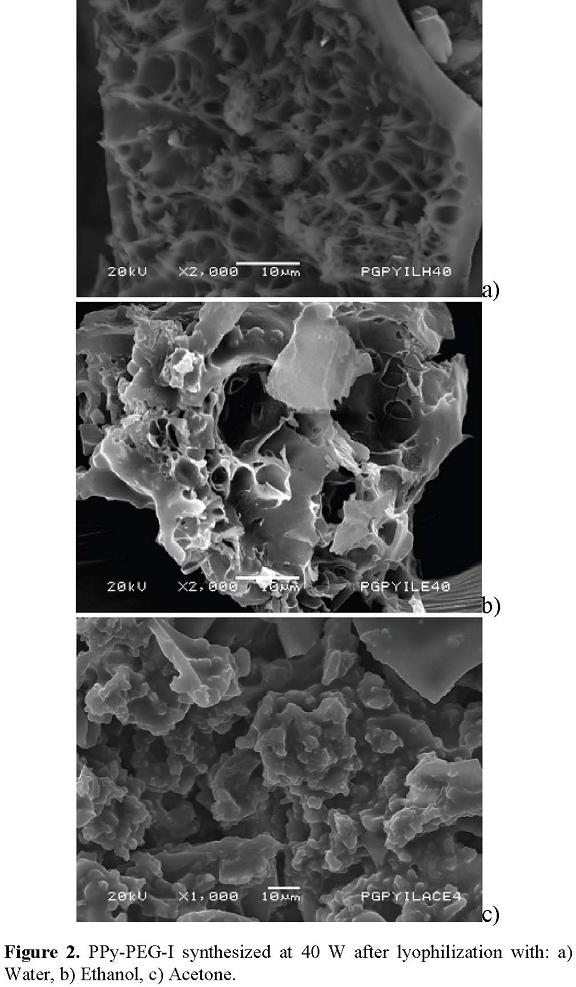
The result was the formation of a great amount of pores of several sizes with partial separation and rupture of layers. Figure 2 shows the morphology of PPy-PEG-I copolymers synthesized at 40 W after the treatment with absorption of water, ethanol and acetone, Figure 2(a), Figure 2(b) and Figure 2(c), respectively. Note the great amount of pores created with the lyophilization. With acetone, the polymers look partially melted, which suggests that this solvent separated a fraction of the material.
3.3 Pore diameter
The evolution of pores was studied following their diameters with a normal distribution function expressed in Equation 1, which in the case of Figure 3 is the width channel. At least 50 measures per graph were used.
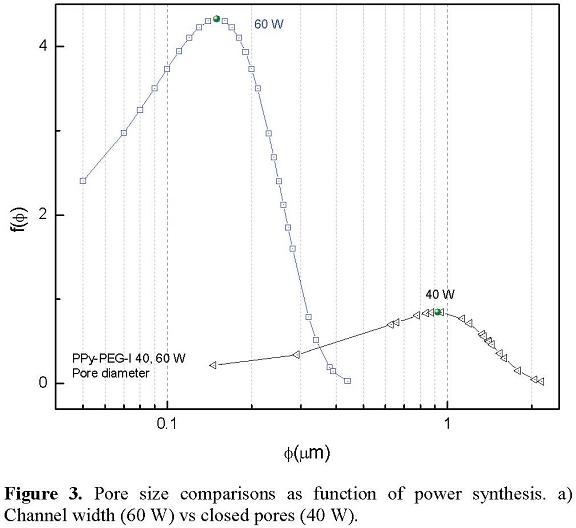

Where: f(φ): normal distribution function, φ: porous diameter, µ: harmonic mean, σ: standard deviation. Figure 3 presents a pore diameter comparison of PPy-PEG-I synthesized at 40 and 60 W in order to evaluate the influence of power in the synthesis. It can be observed that when the power increases, the pore diameter and data dispersion decreases. It should be considered that the data involve different kind of pores; at 60 W porosity is represented by channel width and at 40 W by diameter of closed pores.
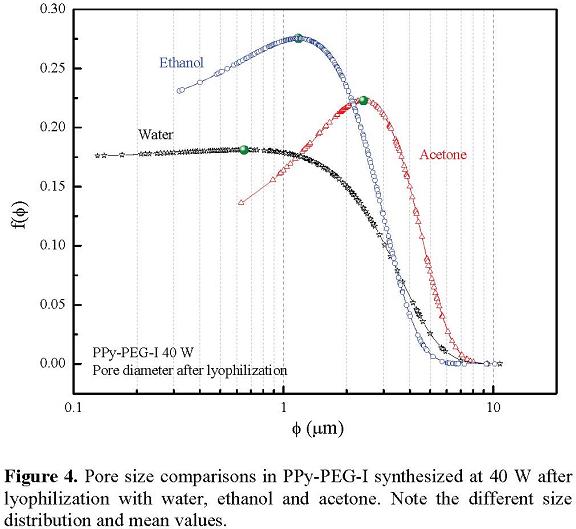
Figure 4 presents a comparison between copolymers synthesized at the same power (40 W) lyophilized with water, ethanol and acetone. With water; the pores in the film have different sizes and shapes located mainly on the surface of the copolymer. The pore size was between 0.13 µm and 10.75 µm, with harmonic mean at 0.65 µm. With ethanol, the pores look interconnected with different sizes distributed randomly in the copolymer. The sizes are greater than those obtained with water, between 0.04 µm and 10.19 µm. Note that ethanol dissolves parts of the copolymer reorganizing the morphology. With acetone, the pores are all over the copolymer without a definite form. Pores sizes range from 0.65 µm to 9.42 µm, with harmonic mean of 2.4 µm.
3.4 Chemical Structure of PPy-PEG-I
Figure 5 shows the IR spectra of PPy-PEG-I synthesized at 40 and 60 W. The spectra has similar absorption bands in both cases. The most intense and wide band is centered at 3425 cm-1 corresponding to N-H, O-H and aromatic C-H bonds. O-H and N-H groups are found in many biocompatible materials. The peak at 2943 cm-1 corresponds to C-H aliphatic groups of ethylenglycol and to the fragments of some pyrrole rings. This absorption is comparatively small compared with the rest of the absorption
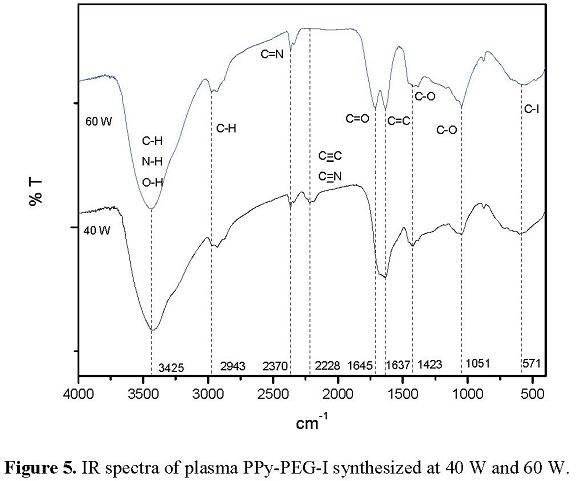
The signal of consecutive double bonds is found at 2370 cm-1, which may be combinations of C=C, C=O and C=N. Multiple bonds are originated from the intense dehydrogenation caused by the high kinetic energy of particles in the plasma. The spectra also show triple bonds that can be found at 2228 cm-1 with two possibilities, C≡C and C≡N.
Non-consecutive double bonds C=C, C=O and C=N appear on the wide absorption centered in 1645 cm-1 for the case of C=O and 2637 for C=C. These bonds are formed by the dehydrogenation of 2 neighboring atoms.
Oxygenation in the copolymers can be observed at 1423 and 1051 cm-1 with the absorption of C-O-H groups. The broad absorption centered at 571 cm-1 corresponds to the complex halogenation of the copolymer with C-I bonds. [913].
All the data above discussed indicate that the copolymers were constructed with the groups of both monomers, pyrrole and ethylenglycol. However, they were dehydrogenated forming new groups with multiple bonds.
4. Conclusions
Plasma copolymers based in ethylenglycol, pyrrole and iodine were obtained as thin, compact and slightly pored films with the purpose to use the copolymer as biocompatible material. The structure of the copolymers has the main groups of both monomers and other new groups with multiple bonds as a consequence of the dehydrogenation promoted by the energy of the plasma.
The diameter of pores was inversely influenced by the power applied during the synthesis. At 60 W, pores have interconnected channeled morphology, but at 40 W, pores were closed, isolated and circular with diameter from 50 to 435 nm. To promote the creation of new pores, after the synthesis the copolymers were lyophilized with water, ethanol and acetone finding that a great amount of new interconnected pores appeared with the treatment with diameter between 0.13 µm and 10.75 µm. The results indicate that apart from its own biocompatibility, this material could increase the interaction with cells with the enlarged surface provided by the pores.
Acknowledgement
The authors thank C. Jorge Perez for the support in the scanning electron microscopy and to Conacyt for the partial support with 80735 and 130190 projects.
References
[1]. D. Xin, Y. Sun, T. Bien, T. Oingfeng, Y. Xiaojun, S. Chengyong, Wei W., Chemical Communications. 46, 970, (2010) [ Links ]
[2]. A. Göpferich, R. Langer. Macromolecules, 26, 4105(1993). [ Links ]
[3]. H. Cheung, K. Lau, T. Lu, D. Hui, Composites: Part B. 38, 291( 2007). [ Links ]
[4]. E. Colín, M.G. Olayo, G.J. Cruz, L. Carapia, J. Morales, R. Olayo. Progress in Organic Coatings. 64, 322 (2009). [ Links ]
[5]. A. Reza, E-Journal of Chemistry, 3, 186(2006). [ Links ]
[6]. B.R. Borgens, R. Shi, The FASEB Journal, 14, 27(2000). [ Links ]
[7]. F. Poncin-Epaillard, G. Legeay. Journal of Biomaterials Science, Polymer Edition. 14, 1005 (2003). [ Links ]
[8]. A. Choukourov, H. Biederman, D. Slavinska, L. Hanley, A. Grinevich, H. Boldyryeva, A. Mackova. The Journal of Physical Chemistry B. 48, 23086 (2005). [ Links ]
[9]. R. Silverstein Spectrometric Identification of Organic Compounds. 7ª. Ed. Wiley. (2005). [ Links ]
[10]. Ninng Yong-Cheng. Structural Identification of Organic Compounds with Spectroscopic Techniques. Wiley-VCH. (2005). [ Links ]
[11]. B. Schrader Infrared and Raman Spectroscopy. Methods and Applications. VCH. (1995). [ Links ]
[12]. G.J. Cruz, M.G. Olayo, O.G. Lopez, L.M. Gomez, J. Morales, R. Olayo Polymer. 51, 4314 (2010). [ Links ]
[13]. J. Morales, M.G. Olayo, G.J. Cruz, R. Olayo. Journal of Polymer Science Part B: Polymer physics. 40, 1850 (2002). [ Links ]














