Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Superficies y vacío
versão impressa ISSN 1665-3521
Superf. vacío vol.25 no.3 Ciudad de México Set. 2012
Coatings by plasmas of pyrrole on nitinol and stainless steel substrates
De Jesús C.1, Cruz G. J.*, Olayo M. G., Gómez L M.2, López-Gracia O. G.
Departamento de Física, Instituto Nacional de Investigaciones Nucleares Apdo. Postal 18-1027, D.F., CP 11801, México. Correspondencia: guillermo.cruz@inin.gob.mx
García-Rosales G.
1Instituto Tecnológico de Toluca Av. Tecnológico s/n, La Virgen, Metepec, Edo. Mex. CP 52140, México.
2Posgrado en Ciencia de Materiales, Facultad de Química, Universidad Autónoma del Estado de México Paseo Tollocan y Colón, Toluca, Edo. Mex., CP 52000, México.
Ramírez-Santiago A.
Departamento de Sistemas Biológicos, Universidad Autónoma Metropolitana Xochimilco Calz. del Hueso 1100, Col. Quietud, Coyoacán, CP 04966, D.F., México.
Ríos L. C.
Departamento de Neuroquímica, Instituto Nacional de Neurología y Neurocirugía Av. Insurgentes Sur 3877, CP 14269, D.F., México.
Recibido: 17 de diciembre de 2011
Aceptado: 12 de abril de 2012
Abstract
Plasma polypyrrole was synthesized on stainless steel and Nitinol (Ni-Ti alloy) substrates in order to obtain polymer-metal compounds with high adhesion. The work has the objective to cover metal surfaces with polymers to reduce the contact of metallic surfaces with biological tissues in human body implants. The metal samples were subjected to RF glow discharges with water vapor and argon to clean, oxidize and erode before the polymer coatings. After that, the synthesis of polymers was carried out by glow discharges during 10 min at 13.56 MHz, 10-1 mbar and 20 W. The results showed that the coating is carried out in consecutive thin layers with the morphology of the metal at the interface. The coated samples were immersed in phosphate solutions (PBS) pH = 7.4 to simulate the conditions of salt concentration in human arteries and veins. The PPy coated metal substrates have rough morphology with resistance to PBS solutions. However, Nitinol samples needed Ar besides water discharges to clean, oxidize and erode.
Keywords: Stents, nitinol, polypyrrole, plasma.
1. Introduction
Stents are small metallic tubular devices of few millimeters diameter with mesh walls which are used in the circulatory system of people with cardiovascular diseases to modify the blood flow and to prevent collapsing of arteries and veins. The stents are introduced with a catheter within the circulatory system and once they arrive to the problem site are expanded to restore the blood flow and to reinforce the artery walls.
However, the contact between metals and bodily fluids may form, as a secondary reaction, blood clots that obstruct circulation worsening the problem few months later after the implant. To avoid this secondary reaction, biocompatible polymers are being tested to form polymeric compatible interfaces between metals and blood, which would reduce the contact of cells with the metallic surfaces. However the union between polymer and metals is not an easy task, because both phases have different characteristics and properties. Additionally, the polymeric layer on the stents should resist the forces of the blood flow inside the circulatory system. The first forces involve the initial insertion of the stent that the polymeric coating must resist.
Polymer-metal compounds as polythiophene-silver-copper (PTh-Ag-Cu) and polyaniline-silver-copper (PAn/Ag-Cu) have been studied to evaluate their biocompatibility with the result that hydrophobicity increases with roughness and the metallic content [1-3]. Other studies about coatings by plasma of polyallylamine films (P-PPA) on 316 L stainless steel stents showed that the high energy in plasma syntheses causes high crosslinking and density of amine groups on the surface [4]. The P-PPA layer was doped with heparin as anticoagulant because the polymer was not considered enough to prevent clotting in arteries [5].
Other similar studies about polymeric layers added to the surface of Nitinol with the objective of using them in stents used oligomeric silsesquioxanes and combinations of polyurethanes to reduce the corrosion of nitinol in long-term implants of stents [6]. The surface modification was done in three steps, electrochemical oxidation, heat treatment and silanisation. The results were the synthesis of rough polymeric layers covering the metallic surface.
As electrochemical techniques usually do not have the sufficient energetic conditions to attach layers of other materials in hard metallic surfaces, other more energetic conditions should be tested in order to obtain a better attachment between polymers and metals in stents. In this work, the bonding of polypyrrole (PPy) with metallic surfaces is studied by plasma with the purpose of using this polymer as a biocompatible layer between metallic stents and cells in the circulatory system.
To achieve this task, the stent should be coated with a biocompatible layer of PPy. Polymers derived from pyrrole have been tested as implants in the central nervous system after spinal cord injuries resulting partially compatible with the neural tissues [7-10]. Considering this characteristic of PPy, this work studies the synthesis by plasma of PPy on metallic substrates with the dimensions of stents and the resistance of the polymer-metal interface under conditions of immersion in biocompatible solutions.
2. Materials and Methods
Small springs and fibers of stainless steel and Nitinol wires used in dentistry were utilized to simulate the components of the mesh walls of stents. The curved surfaces of the wires provide similar surfaces to the stents. Before coating the metallic pieces, the surfaces of Nitinol wires (C:23.36%, O:6.20%, Ti:34.39% Ni:36.03%) and springs of stainless steel (C:22.42%, O:4.14%, Si:1.26%, Cr:10.33%, Fe:61.83%) were washed and cleaned with water and acetone. The compositions shown are in % atomic.
After that, resistive glow discharges were applied with Argon and later with vapor of water to remove residues of organic agents, to oxidize and to prepare the surfaces to receive the polymeric layer. The electric conditions in plasmas form Ar+ ions that energetically collide against the metallic surface causing erosion without chemical interaction. In the next step, in the discharges with water in gas-phase, OH- and H+ ions are formed and impacted against the surface causing little erosion, but due to their high chemical reactivity, they combine with the surface oxidizing and hydrogenating it. This step could be called superficial sensitization. Finally, the monomer and dopant, iodine, were introduced to the reactor, to polymerize and dope attached to the sensitized surfaces.
The cleansing and the synthesis were performed in a 1630 cm3 vacuum tubular glass reactor with stainless steel flanges at 13.56 MHz provided by an Advanced Energy RFX-600 power supply with electrodes of 7 cm diameter and supports of 21.5 cm length. Pressure was in the interval of 0.13-0.7 mbar.
The reagents used were Pyrrole (Aldrich, 98%) high purity Ar and distilled water in the conditioning of metallic substrates. Separate containers were used to the dosage of the reagents. The synthesis of polymers on the metallic surfaces was in thin layers. The wires coated with PPy were submerged in water, acetone and in a PBS solution, sequentially, to test the permanence of the polymeric layer on the metallic surfaces in wet conditions. The PBS solution (NaCl:24 mmol/L, Na2HPO4:10 mmol/L and KH2PO4:3 mmol/L) was utilized to recreate the saline conditions of blood.
3. Results and Discussion
3.1 Polymeric coating
The conditions to obtain a good interface between polypyrrole and stainless steel were plasma oxidation with distilled water at 100 W and 10-1 mbar for 90 min. After that, the polymerization was carried out at the same pressure at 20 W during 10 min. The morphology of the coated substrates is shown in Figure 1. It is a spring with great tension in the structure. The coating follows the surface of the spring without detachments in the curvatures, neither on the rough end surface, see Figure 1. However, the sharp edges show some separate segments, because of the great change of direction. The surface of these segments is probably the most difficult to coat.

However, in the Nitinol wires, the previous conditions showed low polymeric adhesion on the surface with no polymeric attachment in many areas, even thought the wire was a straight piece, without the spring curves of the case before, see Figure 2.

Only in a few regions is the adhesion possible between metal and polymer. It is important to note that the Nitinol surface is smoother and harder than that of stainless steel and the great hardness, the lack of cavities and anchor points may be the cause of the low polymeric adhesion. Another important point to note is that the polymeric layer is not a solid layer, but a collection of thinner layers that grew stacked one over the other. This effect is not visible when the layer has a homogeneous external surface.
The conditions of good adhesion of PPy on Nitinol were developed applying previous erosion with Ar plasma during 30 min and later another 30 min with water plasma oxidation in the interval of 3-7.7 mbar. The polymerization conditions were the same as in the previous case. Figure 3 show the results of these conditions on a nitinol wire covered with PPy. The wire now has a homogeneous polymeric layer on the surface without detachments or bubbles.

3.2 Interaction with solutions
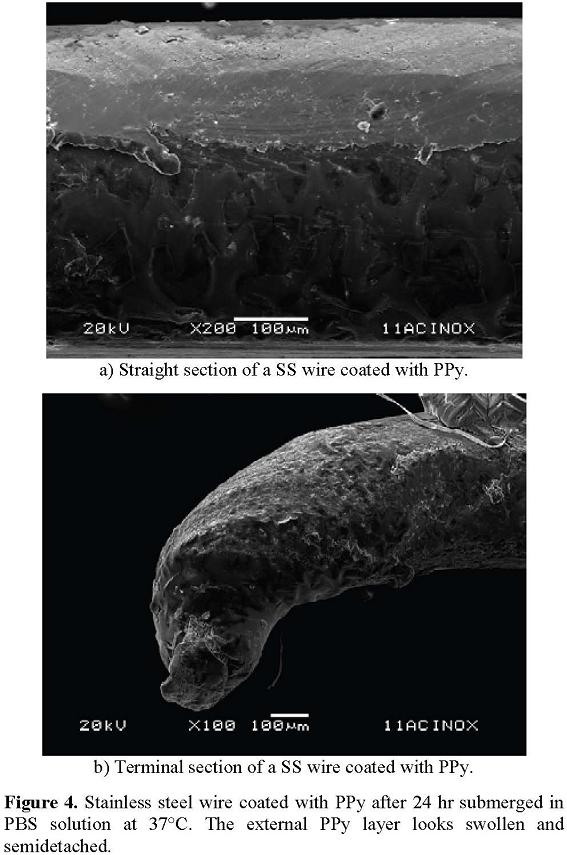
After obtaining smooth polymeric layers on the metallic pieces, they were submerged in the PBS solution described before during 24 hr at 37°C to observe the permanence of the layers in saline solutions with pH and temperature similar to blood. After the treatment with the PBS solution, the polymers appeared still attached to the metallic surfaces, although the layer in direct contact with the solution was swollen and semi detached with some bubbles, see Figure 3. Some of the protrusions observed are clusters of salts that concentrated on the polymer, see Figure 4.
The polymeric coating on Nitinol resisted the PBS thermal treatment a little better, probably because the metallic surface is less rough, see Figure 5. The polymer looks less swollen than in the previous case, although it shows some bubbles and concentration of salts. The coating on the edges is more affected than the smooth areas. The forces applied to cut the wire caused additional tension that affected the polymeric coating in that zone originating more bubbles with the solutions.

4. Conclusions
Several conditions were studied to add PPy coatings by plasma on straight wires and springs of stainless steel and Nitinol with the objective of recreating the coating of metallic stens used as implants in veins or arteries with collapsing problems. The results indicated that the metallic surfaces need to be eroded and oxidized before the polymer is synthesized on the surface. After these two preparation steps, the polypyrrole coatings grew attached to the surface following closely the superficial morphology, although the harder surfaces tend to have less adherence to the polymeric layers.
The formation of consecutive layers may be due to slight changes in pressure during the polymerization and to protrusions on the surface. The coated samples were submerged in phosphate solutions of pH and temperature similar to that of blood in the human system, and generally the polymer remained on the substrates after 24 hr. However there is an accumulation of salts and small bubbles caused by slight polymeric swelling in the edges.
PPy coatings on Nitinol showed a greater resistance to phosphate solutions than those on stainless steel. As the polymers were synthesized at the same conditions, the difference between both may be in the physicochemical conditions of the metallic surfaces that could permeate to the phosphate solutions. As the elements of stainless steel are more reactive than those of Nitinol, the polymeric layers of stainless steel are more sensible to PBS solutions.
Acknowledgements
The authors thank CONACYT for the financial support to this work through projects 80735 and 130190 and to Jorge Perez for the support in the scanning electron microscopy.
References
[1]. J.C. Palacios, G.J. Cruz, M.G. Olayo, J.A. Chávez-Carvayar. Surface and Coatings Technology. 203, 3032 (2009). [ Links ]
[2]. R.S. Langer, N.A. Peppas. Biomaterials, 1, 63 (2002). [ Links ]
[3]. G.J. Cruz, J.C. Palacios, M.G. Olayo, J. Morales, R. Olayo. Journal of Applied Polymer Science. 93, 1031 (2004). [ Links ]
[4]. E. Gallino, S. Massey, M. Tatoulian, D. Mantovani. Surface & Coatings Technology. 205, 2461 (2010). [ Links ]
[5]. Z. Yang, J. Wang, R. Luo, M. F. Maitz, F. Ping, H. Sun, N.Huang. Biomaterials. 31, 2072 (2010). [ Links ]
[6]. R. Bakhshi, A. Darbyshire, J.E. Evans, Z. You, J. Lu, A.M. Seifalian. Colloids Surf B Biointerfaces. 86, 93 (2011). [ Links ]
[7]. R. Olayo, C. Rios, H. Salgado, G.J. Cruz, J. Morales, M.G. Olayo, M. Alcaraz, L. Alvarez, R. Lozano, C. Morales, A. Diaz, Journal of Materials Science: Materials in Medicine, 19, 817 (2008). [ Links ]
[8]. P.M. George, A.W. Lyckman, D.A. LaVan, A. Hegde, Y. Leung, R. Avasare, C. Testa, P.M. Alexander, R. Langer, M. Sur. Biomaterials. 26, 3511 (2005) [ Links ]
[9]. T.F. Otero, M.T. Cortes. Adv. Mater. 15, 279 (2003) [ Links ]
[10].X. Cui, J. Wiler, M. Dzaman, R.A. Altschuler, D.C.Martin. Biomaterials. 24, 777 (2003). [ Links ]














