Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Superficies y vacío
versión impresa ISSN 1665-3521
Superf. vacío vol.25 no.2 Ciudad de México jun. 2012
Thin film composites of polyallylamine-silver
Palacios J. C. *
Facultad de Ingeniería, Universidad Autónoma del Estado de México Cerro de Coatepec, s/n Ciudad Universitaria, Toluca, Edo. Mex., CP 50130, México. *cuauhtemocpalacios@hotmail.com
Olayo M. G., Cruz G. J.
Departamento de Física, Instituto Nacional de Investigaciones Nucleares Apdo. Postal 18-1027, D.F., CP 11801, México.
Chávez J. A.
Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México Circuito exterior, Ciudad Universitaria, Coyoacán, D.F., CP 04510, México.
Recibido: 31 de octubre de 2011
Aceptado: 8 de mayo de 2012
Resumen
Durante la elaboración de materiales biocompatibles deben tomarse en cuenta dos aspectos fundamentales de superficie. El primero es la composición química y el segundo la rugosidad. Estos factores determinarán la naturaleza hidrofílica del material, que es esencial para su aceptación o rechazo por algún organismo vivo. En este trabajo se estudia la morfología e hidrofilicidad de películas de polialilamina y compuestos en película delgada de polialilamina-plata, con el objeto de evaluar si el material será hidrofílico o hidrofóbico, y encontrar condiciones de síntesis para obtener materiales biocompatibles. Por medio de plasmas de resplandor se sintetizaron polímeros con diferente concentración metálica, donde la rugosidad puede ser controlada variando las condiciones de síntesis como presión, composición, flujo de gas y densidad de potencia dentro del reactor. Los resultados muestran superficies con rugosidades nano y micro estructuradas que podrían ser desde hidrofóbicas hasta muy hidrofílicas.
Palabras clave: Polialilamina; Plata; Plasma.
Abstract
Two fundamental elements of a surface must be considered when biomaterials are synthesized, chemical composition and roughness. Both elements will determine the hydrophilic nature of the material, which is essential for their acceptance or rejection in a living organism. In this work, morphology and wettability of polyallylamine-silver thin films synthesized by plasma were studied using microscopic techniques to evaluate their hydrophilic or hydrophobic nature and to find suitable synthesis conditions to obtain acceptable biomaterials. By means of glow discharges, polymers and composites with different metal concentrations were synthesized, where the roughness could be controlled by synthesis conditions, such as pressure, gas composition, gas flow rate and power density inside the plasma reactor. Surfaces with nano and microstructured roughness are observed varying from hydrophobic until hydrophilic nature.
Keywords: Polyallylamine; Silver; Plasma.
1. Introduction
Potentially biocompatible polymers must be able to interact with aqueous media with the possibility to fix proteins and cells in such a way to produce a favourable biological response [1-4]. In this context, the chemical nature of the surface plays a fundamental role in the biocompatibility of materials. For example, superficial amines have been used for immobilization of biomolecules and cell growing. One way to obtain high density of superficial amines is by plasma. Films with different concentration of amines can be obtained with low energy glow discharges using different nitrogenated monomers as precursors [5].
On another hand, biocompatible metals, as stainless steels and Ni and Ti alloys, must be resistant to corrosion and dissolution in biological media. Ag is an excellent electric conductor and although it is a broad-spectrum biocide, in small quantities, it can be combined with polymers to increase the transport of electric charges in composites. Nevertheless, the effect of a metal mixed with polymers goes beyond their electric properties and it can be used to change the surface energy in specific applications, for example, in biomaterials. Many efforts have been made to obtain biocompatible polymer-metal composites with good electrical and hydrophilic properties [5]. On the surface, roughness can significantly modify the biocompatibility of polymers, as essential as its chemical nature. One way to quantify the hydrophilicity of a surface is by measuring the contact angle formed by the surface and drops of different solutions. Hydrophilic surfaces get wet while hydrophobic ones reject the fluids, and the shape of the droplet would be spherical.
The Cassie and Baxter model [6] attempts to describe the wetting of a heterogeneous surface like a liquid-air composite system, which will be largely hydrophobic if the surface becomes rough enough. It has been found that for nano and micro-structured surfaces, large contact angles are measured [7], and on the contrary, smooth surfaces help hydrophilicity.
In this work, the morphology of polyallilamine-silver (PAl-Ag) composites synthesized by plasma is studied to find appropriate synthesis conditions of biocompatible polymer-metal composites. Usually, syntheses by plasma are used to form thin films of materials, which are difficult to obtain by conventional techniques. The vapour phase and the plasma energy can lead to simultaneous polymerization and metal sputtering, which are necessary to form polymer-metal composites. In these conditions the composites are able to combine the biocompatibility of the organic phase and the charge transport of the metal phase.
2. Experimental
The syntheses of PAl-Ag composites were done in a glass cylindrical reactor with stainless steel flanges and flat electrodes at each side. Both, flange and electrode of each side, were maintained at the same electrical potential. The anode was 65 mm diameter made of stainless steel and the cathode was 35 mm diameter made of a 90–10% Ag–Cu alloy. The distance between electrodes was 3 mm. The electrical potential between the electrodes was applied with a power supply (MDX Advanced Energy magnetron drive) operated under constant voltage mode with the output power regulated at 800 V, see Figure 1. A mechanical vacuum pump for corrosive gases Alcatel Pascal 2015C1 was used to reduce the pressure in the system maintaining a base pressure of 0.08 mbar.
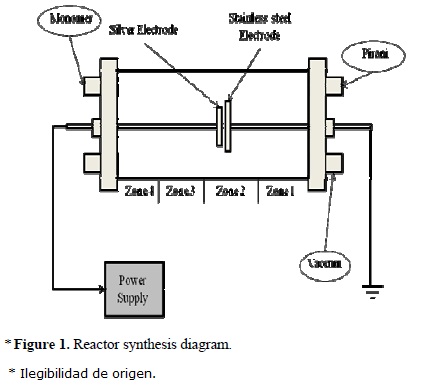
Allylamine (CH2=CH-CH2-NH2, Aldrich 98%) was introduced to the reactor in vapour phase. A flow control valve regulated the monomer feed rate and air until a work pressure of 0.3 mbar was obtained. The amine groups were the reason why allylamine was selected as precursor in the composites. The time of syntheses was 240 min. In these conditions, simultaneously polymerization and metal sputtering were carried out [6]. A high rate of monomer feed could form a layer on both electrodes interrupting the electric discharges, and a low rate of monomer feed provokes a high metal concentration. Thus, the experimental conditions were established considering these restrictions and the samples were obtained in four different zones of the reactor, see Figure 1, where the glow discharges were generated between the anode and the negative flange.
The composites are described in terms of their position in the reactor. Sample 1 was taken in the area between the grounded flange and the electrodes, near the anode. Sample 2 was located between the electrodes. Samples 3 and 4 were obtained between the cathode and the negative flange. Sample 4 was obtained near the negative flange and consequently was the most distant from the electrodes. It has been found that the metallic content in composites is higher nearby the electrodes and decreases with distance [7].
3. Results and discussion
3.1 Morphology
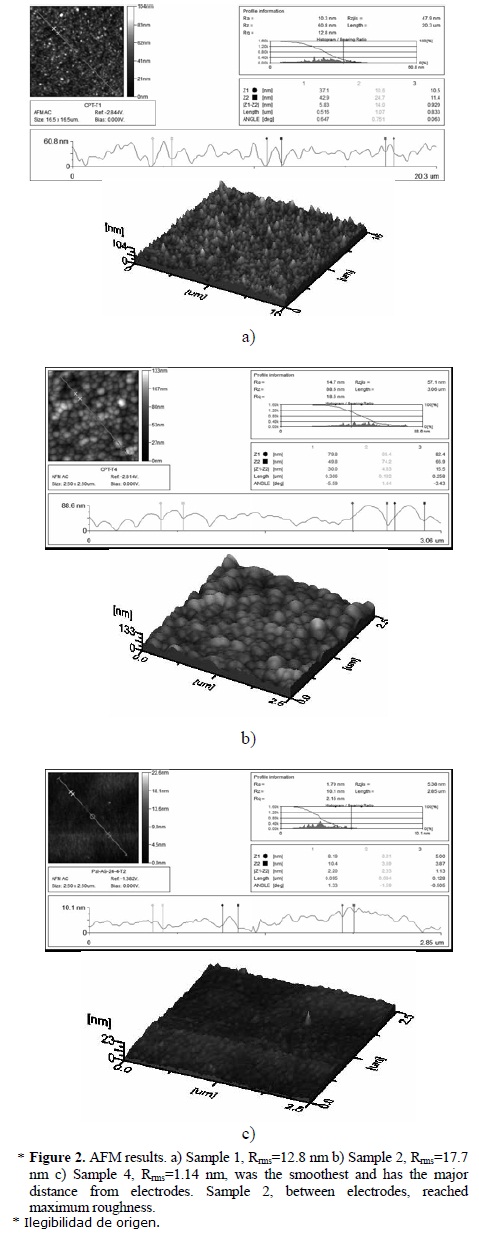
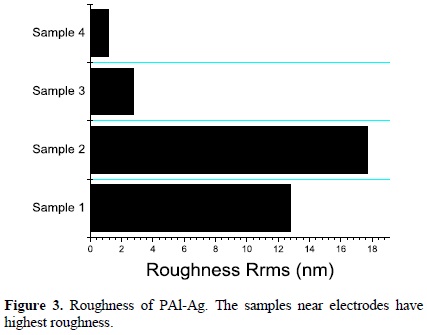
Atomic force micrographs are shown in Figure 2. The highest roughness was found in the Samples 1 and 2, Figure 2(a) and 2(b), with root mean roughness (Rrms) from 12.8 nm to 17.7 nm respectively. This nanoroughness could be due to the frequent impact of the ions with the surfaces surrounding highly energetic zones, especially in Sample 2, placed between the electrodes. The smoothest surface, Rrms=1.14 nm, Sample 4, is the most distant from the electrodes and has the least silver content. In Figure 3 the roughness of each sample is compared. It is evident that the roughness grows with the energy of the plasma particles whereas the polymer practically without metal (Sample 4) is the smoothest sample.
3.2 Contact angle
Contact angles were measured using drops of distilled water at room temperature with three different volumes of 8, 12 and 16 μL. A very hydrophilic surface is one with contact angles lower than 30°. It has been reported that polyallylamine synthesized by rf plasma has contact angles from 13° to 16° [5]. Although the terms hydrophobic and hydrophilic are commonly used, the dividing line between them, for some authors near 90°, it is not well defined. However, highly water repellent surfaces have contact angles over 150° [8].
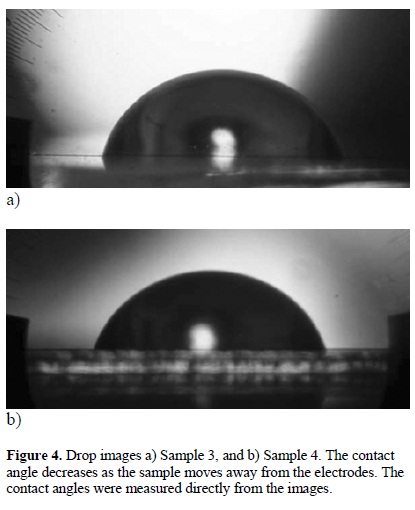
Contact angles from 60 to 113° were measured as a function of the sample position on the reactor, see Figure 4. The contact angle decreases as the sample moves away from the electrodes. From Figure 4(b), the smoothest sample, reached contact angles around 60° whereas the samples between electrodes reached contact angles higher than 113°, which represents an increase of almost 100° above the rf polymers and a moderated hydrophobicity. From Figure 5, it is clear that Sample 2 is the most hydrophobic while the smoothest is the most hydrophilic. Nevertheless, the relation between roughness and contact angles is not completely linear, see Figure 6.
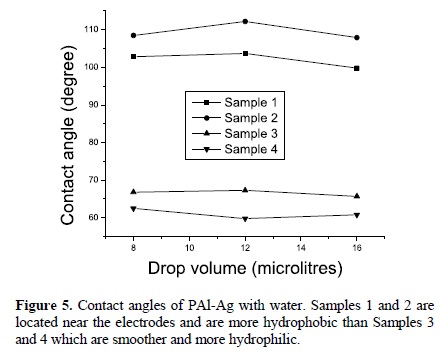
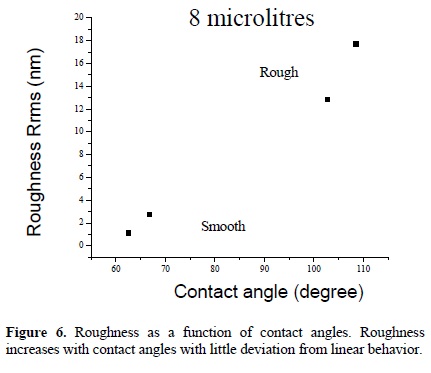
The variation in contact angles has been associated with both, rough and chemical heterogeneities. The last one, results evident from the dissimilar surface tension between polymeric and metallic phases in the composites. Wenzel, and Cassie and Baxter have explained the former. Wenzel proposes a roughness factor, r, to correct the Young equation assuming that the liquid fills up the grooves on a rough surface. Cassie and Baxter assumed that the liquid does not fill the grooves on the rough surface, forming a composite surface with both, the liquid-solid and liquidvapor interfaces. Our experimental results show a little deviation from Wenzel and Cassie and Baxter models. The Cassie and Baxter model predicts a linear relation between the cosine of contact angles and the fraction of solid surface area in contact with the drop. Our deviation can be associated with the modification of the surface tension due to the silver particles in the liquid-solid interface [6].
Neither Wenzel nor Cassie and Baxter models consider chemical changes of the surface. The high surface energy associated with metals increases the surface energy of composites, and consequently despite the fact that high roughness was found, the contact angles could be moderated.
4. Conclusions
By means of simultaneous plasma polymerization and metal sputtering, polymer-metal composites with different metal concentration and roughness were synthesized. The composites were characterized using AFM, whose results showed nano and micro-structured roughness. This morphology influences the surfaces which can be from hydrophobic to hydrophilic. The most hydrophobic samples were obtained in zones of high energy, while the most hydrophilic were found in zones of low energy in the reactor, even with low metal concentration. As a result, the roughness could be controlled by the synthesis conditions, such as pressure, gas composition, gas flow rate and power density, inside the plasma reactor and the wettability depends on the zone were materials are synthesized. In this way, the most hydrophilic composites are in zones of low energy.
Acknowldegement
To Carlos Flores-Morales of IIM-UNAM for his help in the AFM measurements.
References
[1]. R. A. Meyers, C. C. Perry. Encyclopedia of Physical Science and Technology. Biotechnology, (Academic Press, USA, 2001). [ Links ]
[2]. E. Poncin-Epaillard, G. Legeay. J. Biomater. Sci., Polym. Ed. 14 (10), 1005 (2003). [ Links ]
[3]. B. D. Ratner. J. Mol. Recogn. 9, 617 (1996). [ Links ]
[4]. T. Otero, M.T. Cortez. Adv. Mater. 15, 279 (2003). [ Links ]
[5]. E. Colín, M. G. Olayo, G. J. Cruz, L. Carapia, J. Morales, R. Olayo. Prog. Org. Coat. 64, 322 (2009). [ Links ]
[6]. G. J. Cruz, J. C. Palacios, M. G. Olayo, J. Morales, R. Olayo, J. Appl. Polym. Sci. 93, 1031 (2004). [ Links ]
[7]. J. C. Palacios, G. J. Cruz, M. G. Olayo, J. A. Chávez- Carvayar. Surf. Coat. Technol. 203, 3032 (2009). [ Links ]
[8]. L. Zhang, H. Chen, J. Sun. J. Shen, Chem. Mater. 19, 948 (2007). [ Links ]














