Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Superficies y vacío
versão impressa ISSN 1665-3521
Superf. vacío vol.25 no.1 Ciudad de México Mar. 2012
Surface modification of poly(tetrafluorethylene) magnetic stirring bars with plasma of hexamethyldisiloxane and its applications in the stir bar sorptive extraction technique
Baeza–Marrufo R.1, Acereto–Escoffié P.1, Carrera–Figueiras C.1, Muñoz–Rodríguez D.1*, Ávila–Ortega A.1, López–Barrera J. A.2, Morales–Corona J.3, y Olayo–González R.3
1 Universidad Autónoma de Yucatán, Facultad de Ingeniería Química, Periférico Norte Km 33.5. Tablaje Catastral 13615. Col Chuburná de Hidalgo Inn C.P. 97203. Mérida, Yucatán, México.*david.mr@uady.mx.
2 Academia de Física, Universidad Autónoma de la Ciudad de México La corona # 320, Col. Loma la palma . DF., CP. 07160, México D.F.
3 Departamento de Física, Área de Polímeros, Universidad Autónoma Metropolitana, Unidad Iztapalapa Av. San Rafael Atlixco 186, Col. Vicentina, 09340, D.F., México.
Recibido: 31 de mayo de 2011;
Aceptado: 26 de enero de 2012.
Abstract
This paper explores the potential of plasma polymerization to modify the surface of stir bars for its use in Stir Bar Sorptive Extraction (SBSE). The modification of the poly(tetrafluoroethylene) surface of stir bars was done through plasma polymerization of hexamethyldisiloxane (30 min, 110 W and 1.4×10–1 Torr). The coating was characterized by contact angle, Fourier Transform Infrared Spectroscopy, Energy Dispersive X–ray Analysis and Scanning Electron Microscopy. The modified stir bars were applied in SBSE to extract five organophosphorus pesticides (dichlorvos, diazinon, dichlofenthion, malathion and fensulfothion) from synthetic aqueous solutions. The extracts were analyzed by gas chromatography with mass spectrometric detection. The modified bars adsorbed four pesticides (stirring 60 min, back extraction with 1.4 mL of isooctane and 15 min of sonication).
Keywords: Gas Chromatography; Stir bar sorptive extraction; Pesticides; Plasma polymerization; Hexamethyldisiloxane.
1. Introduction
Stir bar sorptive extraction (SBSE) was developed and introduced in 1999 by Baltussen et al as an environmental friendly technique for analytical preparation of aqueous samples [1]. In SBSE, a film of polydimethylsiloxane (PDMS) is deposited on a magnetic stirring bar coated with glass. The stir bar is introduced into an aqueous sample and subjected to agitation to facilitate the sorption of analytes into the PDMS [2]. Then, the analytes can be desorbed by thermal (TD) or liquid desorption (LD) once they have reached the equilibrium between the polymer and the aqueous phase. In TD the stir bar is removed from the extraction reservoir and introduced into a thermal desorption unit installed in a gas chromatograph (GC). In the LD the stir bar is immersed in an appropriate solvent to elute the analytes and then the solvent is introduced into a GC or high performance liquid chromatograph (HPLC) [35].
SBSE has been applied for trace analysis of a wide variety of organic compounds (polyaromatic hydrocarbons, PCBs, pesticides, preservatives, steroids, estradiol, etc.) in environmental samples (usually water), beverages (coffee, beer, and whiskey) and biomedical samples (urine, plasma and breast milk) [1, 6–8].
The most widely used coating in SBSE is a non–polar polymer, polydimethylsiloxane (PDMS). Consequently, PDMS has shown good recoveries for non–polar analytes but recoveries decrease for polar analytes [8]. Thus, one of the challenges is to prepare SBSE coatings for the extraction and pre–concentration of analytes with different polarity. For this purpose, different in–house procedures have been reported.
Stir bars with PDMS coating have been prepared by the sol–gel technique and applied to the extraction of n–alkanes, polyaromatics (PAHs) and organophosphorus pesticides (OPPs) [9,10]. Additionally, different chemical groups like ß–cyclodextrin [11,12], divinylbenzene (DVB) [13] and poly(vinyl alcohol) [14] have been introduced in the PDMS network by sol–gel to extract estrogen and bisphenol A. However, it has been reported that the polymer layer cracked and loss over time [8].
Furthermore, sorbents based in monolithic materials have been obtained by polymerization of a monomer mixed with a porogen solvent [15,16]. The coatings obtained include various mixtures of monomers like octyl methacrylate–ethylene dimethacrylate [17], methacrylic acid stearyl ester [18], methacrylic acid stearyl ester–ethylene dimethacrylate [19], vinylpyrrolidone–divinylbenzene[20] ,vinylimidazole–divinylbenzene [21], vinylpyridine–ethylene dimethacrylate [22]. Also, coatings of poly (phthalazine ether sulfone ketone) were used to extract organochlorine and OPPs from sea water and juices by SBSE [23]. Polyurethane foams have been proposed as polymeric phases to extract polar analytes by SBSE [24].
In addition, more selective stir bars based on restricted access materials (RAM) and molecular imprinted polymers (MIP) have been synthesized and evaluated [25, 26]. However, there are no reports about the application of plasma polymerization to develop coatings on stir bars for SBSE.
Surface modification or polymer coatings have been obtained by plasma polymerization in a variety of substrates with different geometries [27, 28]. The materials obtained are significantly different from the polymers produced by conventional techniques. Plasma polymers may have a heterogeneous and highly crosslinked composition depending on the polymerization conditions used, thereby generating a variety of materials with physical and chemical characteristics very different from each other even using the same monomer [29–33]. This heterogeneous composition would favor the adsorption of organic molecules with different degrees of polarity with an adjustment of polymerization conditions and the type of monomer used.
In this context, the aim of this paper was to modify the PTFE surface of stir bars used in SBSE through plasma polymerization of HMDS. The coating was characterized by Scanning Electron Microscopy (SEM), Energy Dispersive X–Ray Analysis (EDAX), Fourier Transform Infra–Red (FT–IR) and contact angle. As an additional test, the modified stir bars were used to extract five OPPs from synthetic aqueous solutions and the extracts analyzed by GC–MS.
2. Experimental
2.1. Reagents
The monomer hexamethydisiloxane was purchased from Aldrich (+ 98%). The isooctane and isopropanol were obtained from J. T. Baker (Mallinckrodt Baker, Phillipsburg, NJ, USA). The standards of pesticides [dichlorvos (DCV), diazinon (DZN) dichlofenthion (DCF), malathion (MLT), fensulfothion (FST) ] were ChemService brand (West Chester, PA, USA). The purity of all standards was always higher than 98.5%. Individual stock solutions of all pesticides were prepared in isopropanol at a concentration of 100 ppm and stored at –18 ° C. A working solution containing all compounds studied at a concentration of 1 ppm in water was previously prepared from each pesticide stock solution.
2.2. Plasma surface modification of magnetic stirring bars with hexamethydisiloxane
For the generation of plasma, a reactor consisting of a glass tube was used (25 cm × 10 cm id). The tube was coupled to a pair of internal capacitive electrodes that were connected to a generator of radio frequency (13.56 MHz) through impedance coupling both of Advanced Energy brand. Hexamethydisiloxane monomer was fed to the reactor through a pressure difference between the reactor and its container. The vacuum inside the reactor was achieved using a vacuum pump model 2015 Adixen brand C2.
Polymerizations were carried out by plasma of hexamethydisiloxane (30 min, 100 W, 4.5×10–1 Torr), on the magnetic stirring bars (12.7 × 3.5 mm, Cienceware from Bel–Art Products) pretreated with air plasma (1 h, 120 W, 5×10–1Torr). Finally, after the plasma polymerization, the power was switched off and the modified stirring bars were left exposed for 20 minutes under the monomer's vapor. This exposition to monomer's vapor is performed in order to end–cap the reactive species located at the bar surface to react with the monomer. In this way the polymer layer deposited onto the bar's surface will not react with the atmosphere.
2.3. Characterization ofplasma polymer film of hexamethydisiloxane, PPHMDS
For SEM analysis, PTFE surfaces unmodified and modified with PPHMDS were coated with gold and then micrographs were taken with a scanning electron microscope (SEM) JEOL JMS 6360LV (accelerating voltage, 20kV). Elemental microanalysis was performed on PTFE surfaces unmodified and modified with hexamethydisiloxane plasma using an EDAX (Oxford Instruments, INCA Energy 200) attached to SEM. For the analysis of FT–IR, KBr pellets were placed adjacent to the stir bar. The spectra of PPHMDS were obtained in a FT–IR spectrophotometer Nicole Protects 460 in transmission mode with 30 scans with a resolution of 4 cm–1. Contact angles were measured with a drop of water on PTFE surfaces unmodified and plasma modified by hexamethydisiloxane. The images of the contact angles were taken with a camera BENQ DC.C840 and analyzed with ImageJ software [28].
2.4. Extraction SBSE
The stirring rods modified with PPHMDS films were used in the SBSE extraction of aqueous solutions spiked with pesticides. Bars were immersed in Erlenmeyer flasks containing 10 mL of pesticides in aqueous solution (1 µg·mL–1) and stirred for 60 min. Then, the stirring bar was removed from the solution and dried with a lint–free cloth. After that, the bars were placed in microtubes (1.5 mL) and the retained compounds were desorbed by addition of 1.4 mL of isooctane in order to cover the bars completely. Next, the microtubes were placed in an ultrasonic bath for 15 min, and finally, the stir bar was withdrawn and the solvent was analyzed by GC–MS.
2.5. Chromatographic analysis by GC–MS
Pesticide extracts were analyzed in an Agilent Technologies 6890N gas chromatograph coupled with a mass spectrometer 5973 N. For pesticide separation, a fused–silica column Equity–5™ (5% phenyl–95% polydimethylsiloxane; 30 m × 0.25 mm ID, 0.25 μm), supplied by SUPELCO was used. The carrier gas was He at flow rate of 1 mL–min–1. 1μL of extract was injected into a programmed temperature vaporization (PTV) injector at 250° C in the mode splitless. The column temperature was programmed as follows: 120° C for 3 min then increased to 280° C at 30° C • min–1 and holding for 3 min.
The typical operating conditions of MS were optimized with the software autotune option. The electron impact mode (70 eV) was used as ionization source (250° C) and ion masses were monitored between 50–400 m/z. The temperature of the ion source and quadrupole were 250° C and 150° C, respectively. Organophosphorous pesticides used as model analytes were identified by comparing their mass spectra with mass spectra of the electronic library (NIST 98). Additionally, the MS was used in SIM mode (selected ion monitoring). The ions monitored were selected according to literature and considering the mass spectra obtained from chromatograms recorded in the SCAN mode. The target (T) and qualifier (Q1, Q2) ions used in the SIM program are shown in Table 1.
3. Results and Discussion
3.1. Modification of magnetic stirring bars with PPHMDS films
The modification of PTFE surfaces as a result of the action of plasma is attributed to bombardment of the substrate by a great variety of particles as electrons, ions, free radicals, etc. The result of air plasma pretreatment was to clean the surface at molecular level and to create active sites through breakage and rearrangement of chemical bonds. In consequence, the activated surface can react with active gas (oxygen and nitrogen) to modify the surface chemistry with the introduction of functional groups such as nitriles, peroxides, etc. As a result, surfaces may be more susceptible to interact with the active species of the monomer. This is particularly useful for a good adhesion between the polymeric film and the substrate.
3.2. Morphological analysis of PPHMDS films
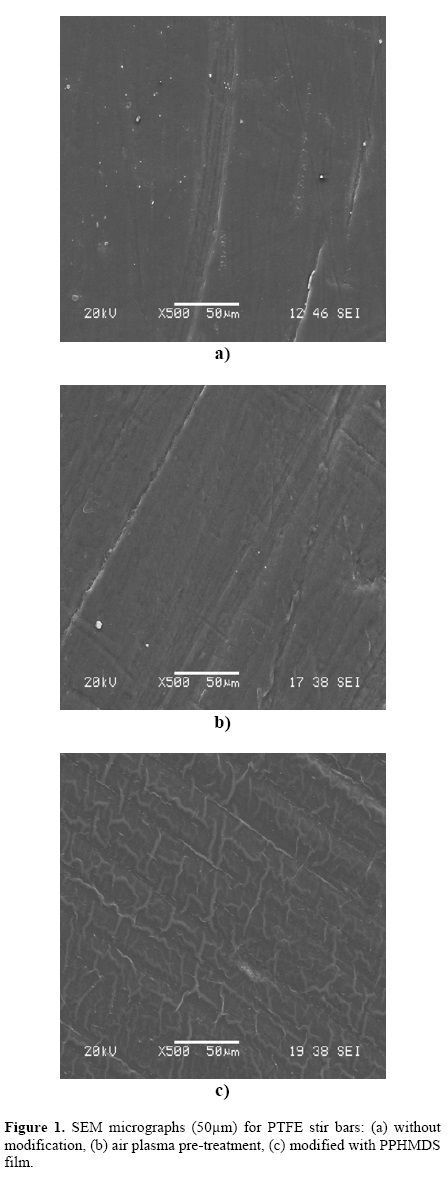
The figure 1 shows the SEM micrographs of PTFE surfaces. Figure 1b shows the erosion of the activated PTFE surface as a result of pre–treatment with air plasma. Figure 1c shows the presence of rough PPHMDS films synthesized on the surface of PTFE. The films measured with a Mitutoyo Co. micrometer had a thickness of 0.01 mm. It has been reported that the hexamethydisiloxane and other monomers make different arrangements when they are polymerized by plasma, where the affinity of the substrate and the polymer formed play an important role in the shape of the polymer layer. Stratman et al [33] have observed that hexamethydisiloxane forms a rough film very similar in morphology to that obtained in this work when using PTFE as a substrate.
3.3. Composition analysis of PPHMDS films
The results of the chemical composition analysis of the unmodified PTFE surface showed that the atomic weight percentage of carbon and fluorine were 18.2% and 81.18%, respectively. This is in agreement with the typical stequiometric ratio for this polymer (4.5). However, the surface activated with air plasma (1 h 120 W) presented a slight decrease in the percentage of fluorine atoms (80.6 %, F/C = 4.2) due its loss during the erosion which took place during the activation of the surface of PTFE. This behavior is similar to the results of Wong et al, where it was observed in XPS analysis a loss of fluorine atoms from the PTFE surface using argon plasma under milder conditions of reaction (28 W, 60 s, 0.15 Torr) [34]. However, in this work despite using air plasma during pre–treatment we did not find oxygen onto the pre–treated surface of PTFE. This was probably due to the high energy that was used (120 W) favoring erosion rather than the incorporation of oxygen functional groups. Indeed no oxygen in PTFE surfaces has been observed even when working with plasma of a controlled flow of oxygen with long curing times (more than 30 minutes) and using relatively high power of 100 W [34].
EDAX results of the modified PTFE surface shows the presence of carbon (17.3 %), fluorine (77.6 %), oxygen (3.7 %) and silicon (1.4 %) in atomic weight percentage. The last two elements are the products of the plasma polymerization of hexamethyldisiloxane.
3.4. FT–IR analysis of PPHMDS films
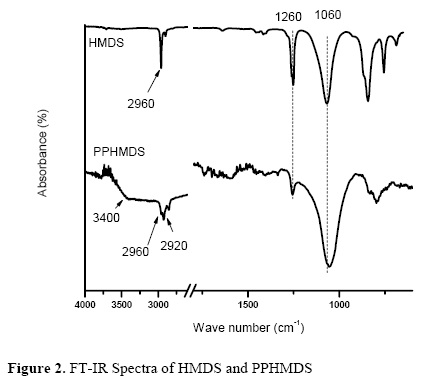
The FT–IR spectra of hexametildisiloxane monomer and PPHMDS (Figure 2) exhibited bands related to Si–C rocking vibrations in Si(CH3)n group displayed in the range of 760–870 cm–1 . These bands are useful to characterize the presence of monomethyl–, dimethyl–, trimethyl–substituted silicon. Clear–cut bands related to the asymmetrical stretching vibration of Si–O–Si group at 1060 cm–1 were presented in monomer and in PPHMDS. In addition, several bands belonging to methyl group bonded to silicon were also present in both spectra. The most obvious are the asymmetrical and symmetrical deformation of methyl group in Si(CH3)n group at 1260 cm–1. The bands of the symmetrical and asymmetrical C–H stretching in methyl group at 2920 cm–1 and 2950 cm–1 were also presented and they are characteristic of methyl group bonded to silicon. These results explain the contact angle values obtained by the hydrophobic nature of surfaces modified by the presence of CH2, Si–CH3 and Si–O–Si groups. During the polymerization process, these structures presented in the monomer spectrum were preserved in PPHMDS coatings however there is a loss of Si–CH3 groups due the power of the electrostatic discharge. For a long time of polymerization (more than 60 min), Dai [35] and Olayo [28] observed that the organic part (Si–(CH3)n) of PPHMDS films was lost and the inorganic part of the film (Si–O–) remained in the film. Even minor contact angles could be generated when surfaces have less Si–CH3 groups. The spectrum of PPHMDS synthesized has a broad band around 3400 cm–1 for the OH group stretching. The presence of these groups is ascribed to the decomposition of HMDS under plasma discharge.
Some polymer coatings obtained by plasma could present –OH groups even when using monomers without oxygen. This occurs when plasma modified surfaces are exposed to air immediately after the polymerization has finished.
Evidence in support of cross–linking and double bond formation on plasma–treated PTFE surfaces has been reported. The surviving radicals which have not reacted in the plasma reactor would be attacked immediately by oxygen and oxygen–containing species as soon as the film is brought into contact with the atmosphere.
For this reason plasma treated surfaces were exposure to monomer atmosphere in order to cure the surface only with monomer before the reactor was opened. Some authors [36] have found that the chemical modification of the PTFE surface is very dependent on polymerization conditions.
3.5. Contact angle measurements of water on PPHMDS films
The water contact angles on PTFE obtained without any treatment, and with pretreatment (120 W, 5 ×10–1 Torr, 60 min) were 54° and 60°, respectively. The contact angle obtained on the modified surface plasma with PPHMDS films (100 W, 1.4 ×10–1 Torr, 30 min) was 80°. The plasma pre–treatment on PTFE slightly increased the contact angle with respect to the Teflon surface without treatment. This behavior has been observed when Teflon and other polymeric substrates are exposed to a plasma discharge. This increase is generally associated with the generation of an eroded surface that causes an increase in the apparent contact angle.
Yasuda has proposed that the operating conditions of gas plasma treatments depend on the composite parameter W/F, where W is the power level of the plasma, F is the gas flow rate, which represents the energy input per molecule [27]. Plasma treatments can be carried out with the control of two conditions in order to get a polymer film: (i) power, (ii) flow rate. Although the flow rate of gas and the level of power supply remained constant in this work, the polymerization conditions were sufficient to generate a plasma polymer film of PPHMDS which modified the surface of PTFE. Low levels of power would probably have meant a low energy per particle and insufficient number of active species. On the other hand, if the power had been too high it could have resulted in undesirable side reactions that break the monomer and the polymer on the surface of PTFE.
The contact angle of PTFE modified by PPHMDS film was 20° greater than the angle measured onto the unmodified PTFE and this could be ascribed to the presence of methyl siloxane groups on the bar's surface.
3.6. Extraction of OPPs with magnetic bars coated with PPHMDS
The coated stirring roads were used to extract five OPPs (1 μg.mL–1) from aqueous solutions by SBSE. These compounds were selected as model analytes because they are widely used in agriculture and are typical endocrine disruptors.
The experiments were carried out separately with four stir bars modified at the same time in the plasma reactor. For comparative purposes, a magnetic stirring bar without modification to extract pesticides from an aqueous solution (1 µg·mL–1) was used. The extraction and desorption conditions were selected according to typical conditions reported in literature for the extraction of organophosphorous pesticides from aqueous samples [2]. Since organophosphorous pesticides are non–ionizable compounds in aqueous solutions and the pH has little effect on the extraction efficiency, this was carried out in neutral conditions. The ionic strength of the solution was not changed and organic modifier was not added. Table 2 presents physicochemical data of analytes and analytical signal of the five pesticides after extraction by SBSE with stirring bars unmodified and modified with PPHMDS.
The table 2 shows the analytical signal (chromatographic peak area) of five pesticides studied after extraction by SBSE with unmodified and modified stir bars. Diazinon, dichlofenthion and malathion showed relatively low signals in extracts where unmodified SBSE stir bar was used. In particular, the, dichlofenthion's analytical signal showed the highest intensity because it is the least polar of the five pesticides and therefore with high affinity for PTFE. With the unmodified stir bar no signals were detected in extracts for dichlorvos and fensulfothion because both have relatively low values of log Ko/w and therefore a greater affinity towards the aqueous phase.
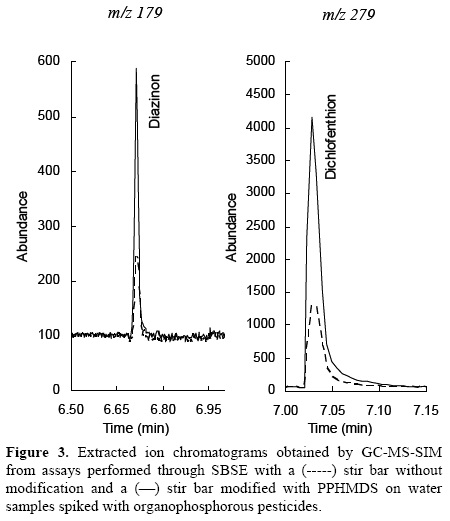
On the other hand, the analytical signal obtained with modified stir bars was higher for four of the five pesticides studied (Table 2). Analytical signal of malathion presented a small increment. In contrast, peaks of diazinon and dichlofenthion were three times higher than their signal obtained with unmodified stir bar (Figure 3). This is because the stir bar's surface, hydrophobicity, facilitates the interaction with fewer polar pesticides (Table 2). Fensulfothion's chromatographic peak was also detected. Nevertheless, there was no signal of dichlorvos because of their relatively high polarity and water solubility.
Recoveries of diazinon and dichlofenthion were low (<1%). This is ascribed to the small amount of the polymer layer (3.9 μL) onto the PTFE bars in contrast to the relatively high concentration of pesticide. Furthermore, some authors reported low recoveries of pesticides when the isooctane was used in liquid desorption with stir bars covered with typical volumes of PDMS (47 μL) [37]. In addition, precision of the analytical signal (RSD < 15%) was relatively good despite the small thickness of the PPHMDS film (0.01 mm). This suggests that the hexamethydisiloxane was distributed uniformly on the four bars modified simultaneously.
Currently, we are developing experiments with magnetic glass rods coated with HMDS plasma polymer films with a thickness similar to that of commercial coatings (0.05 – 1 mm), in order to be used in SBSE technique. Factors that affect extraction and liquid desorption will be studied with diverse pesticides as model analytes.
4. Conclusions
Thin films of plasma polymer of hexamethyldisiloxane (PPHMDS) were synthesized onto the surface of PTFE magnetic stirring bars. These bars were applied in SBSE and extracts analyzed by GC–MS. Results showed that modified bars adsorbed diazinon, malathion, dichlofenthion and fensulfothion. Chromatographic peaks of diazinon and dichlofention were three times higher than their signal obtained with unmodified stir bars. In contrast no signal detection was found for the more polar pesticide, dichlorvos. The intensity of the analytical signal was related with the octanol–water partition coefficient (log K o/w).
Acknowledgements
This research has been supported by the "Programa de Mejoramiento al Profesorado" (PROMEP 103.5/08/2988). The authors would like to thank Dr. Manuel Cervantes (CICY) for the SEM pictures and also Ms. Maria Guadalupe Palafox and Mr. Daniel Joseph for helping us with the translation.
References
[1]. E. Baltussen, P. Sandra, F. David, C. Cramers, J. Microcol. Sep. 11, 737 (1999). [ Links ]
[2]. F. Sánchez–Rojas, C. Bosch–Ojeda, J. M. Cano–Pavon, Chromatrographia 69, 79 (2009). [ Links ]
[3]. A. Prieto, O. Zuloaga, A. Usobiaga, N. Etxebarria, L.A. Fernandez, C. Marcic, A. de Diego, J. Chromatogr. A 1185, 130 (2008). [ Links ]
[4]. R. Rodil, M. Moeder, J. Chromatogr. A 1178, 81 (2008). [ Links ]
[5]. R. Rial–Otero, E.M. Gaspar, I. Moura, J.L. Capelo, Talanta 71, 503 (2006). [ Links ]
[6]. F. David, P. Sandra, J. Chromatogr. A 1152, 54 (2007). [ Links ]
[7]. M. Kawaguchi, R. Ito, K. Saito, H. Nakazawa, J. Pharm. Biomed. Anal. 40, 500 (2006). [ Links ]
[8]. A. Prieto, O. Basauri, R. Rodil, A. Usobiaga, L.A. Fernandez, N. Etxebarria, O. Zuloaga, J. Chromatogr. A 1217, 2642 (2010). [ Links ]
[9]. W. Liu, Y. Hu, J. Zhao, Y. Xu, Y. Guan, J. Chromatogr. A 1095, 1 (2005). [ Links ]
[10]. W. Liu, H. Wang, Y. Guan, J. Chromatogr. A 1045, 15 (2004). [ Links ]
[11]. Y. Hu, Y. Zheng, F. Zhu, G. Li, J. Chromatogr. A 1148, 16 (2007). [ Links ]
[12]. C. Yu, B. Hu, J. Chromatogr. A 1160, 71 (2007). [ Links ]
[13]. C. Yu, Z. Yao, B. Hu, Anal. Chim. Acta 641, 75 (2009). [ Links ]
[14]. C. Yu, B. Hu, J. Sep. Sci. 32, 147 (2009). [ Links ]
[15]. X. Huang , D. Yuan, J. Chromatogr A 1154, 152 (2007). [ Links ]
[16]. X. Huang, J. Lin, D. Yuan, J. Chromatogr. A 1217, 4898 (2010). [ Links ]
[17]. X. Huang, N. Qiu, D. Yuan, Q. Lin, J. Chromatogr. A 1217, 2667 (2010). [ Links ]
[18]. X. Huang, D. Yuan, B. Huang, Talanta 75, 172 (2008). [ Links ]
[19]. X. Huang, N. Qiu, D. Yuan, (2008) J. Chromatogr. A 1194: 134–138. [ Links ]
[20]. X. Huang, N. Qiu, D. Yuan, B. Huang, (2009) Talanta 78: 101–106. [ Links ]
[21]. X. Huang, N. Qiu, D. Yuan, Q. Lin, J. Chromatogr. A 1216, 4354 (2009). [ Links ]
[22]. X. Huang, J. Lin, D. Yuan, R. Hu, J. Chromatogr. A 1216, 3508 (2009). [ Links ]
[23]. W. Guan, Y. Wang, F. Xu, Y. Guan, J. Chromatogr. A 1177, 28 (2008). [ Links ]
[24]. N.R. Neng, M.L. Pinto, J. Pires, P.M. Marcos, J.M.F. Nogueira, J. Chromatogr. A 1171, 18 (2007). [ Links ]
[25]. J.–P. Lambert, W.M. Mullett, E. Kwong, D. Lubda, J. Chromatogr. A 1075, 43 (2005). [ Links ]
[26]. X.L. Zhu, J.B. Cai, J. Yang, Q.D. Su, Y. Gao, J. Chromatogr. A 1131, 37 (2006). [ Links ]
[27]. H. Yasuda, Q. Yu. Plasma Chem. Plasma Process. 24, 325 (2004). [ Links ]
[28]. J.A. López–Barrera, A. Avila–Ortega, J. Morales–Corona, J. Cervantes, R. Olayo, Appl. Organomet. Chem. 21, 858 (2007). [ Links ]
[29]. B. Bae, B.–H. Chun, H.–Y. Ha, I.–H. Oh, D. Kim, (2002) J. Membr. Sci. 202: 245–252. [ Links ]
[30]. H. Kobayashi, A. T. Bell, M. Shen, Macromolecules 7, 277 (1974). [ Links ]
[31]. S. H. Lee, D. C. Lee, Thin Solid Films 325, 83 (1998). [ Links ]
[32]. E. Radeva, Sens. Actuators, B 44, 275 (1997). [ Links ]
[33]. N. Shirtcliffe, P. Thiemann, M. Stratmann, G. Grundmeier, Surf. Coat. Technol. 142, 1121 (2001). [ Links ]
[34]. K.L. Tan, L.L. Woon, H.K. Wong, E.T. Kang, K.G. Neoh, Macromolecules 26, 2832 (1993). [ Links ]
[35]. X. Dai, J. Church, M. Huson, Plasma Process. Polym 6, 139 (2009). [ Links ]
[36]. M. Morra, E. Occhiello, F. Garbassi, Langmuir 5, 872 (1989). [ Links ]
[37]. P. Serodio, J.M.F. Nogueira, Anal. Bioanal. Chem. 382, 1141 (2005) [ Links ]
[38]. L. Brossa, R.M. Marce, F. Borrull, E. Pocurull, Chromatographia 61, 61 (2005). [ Links ]














