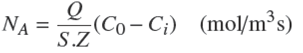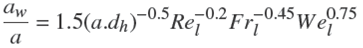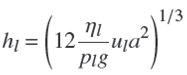1 Introduction
Population growth and development industry and technology are factors that increase environmental pollution such as production of wastewater and greenhouse gases, therefore investment must be done in treatment and disposal of that. That is why a lot of studies and methods have been done for wastewater treatment and removal of dangerous materials like Biological methods (Gan et al., 2013, Gupta et al., 2015), use of adsorbents (Bello-Huitle etal., 2010, Martín-González., 2013) and other methods(Lopez- Ojeda et al., 2015, Staehelin., 1984, Shen et al., 2006 ). Gas absorption such as CO2 capture is one of the most important processes in the chemical industry. Reducing the emission of CO2, which considered a greenhouse gas, has found great importance in recent years. Lopes et al (Lopes et al, 2014) studied the absorption rate were investigated. They found that the absorption rate increases at the beginning of the experiments with low TEA concentration but it decreases with increasing of TEA concentration. To enhance the physical absorption of CO2, an ionic liquid (IL), 1-butylimidazole hexafluoride phosphate [bmim] PF4, was dispersed in surfactant solution to form an IL-in-water emulsion by Liu et al (Liu et al.,2013). Their results indicated that the dispersed IL droplets can significantly increase the absorption rate of CO2 at the gas-liquid interface.
One of the general goals of scientists and engineers is to increase efficiency and miniaturize the systems used in the industry. Nanotechnology has enabled us to make nanoparticles with different types and sizes. Heat transfer nanofluids were first reported by Choi (Choi, 1995). Nanofluid is a new fluid, in which particles with diameter smaller than 100 nm are suspended in the base fluid. Recently, many studies have been carried out on increasing mass transfer by adding nanoparticles. However, the number of these studies is still less than the ones performed on heat transfer and thermal conductivity. Increasing the mass transfer coefficient increases efficiency and reduces size and cost of the equipment. In general, there are three methods for enhancement of mass transfer; mechanical treatment, chemical treatment, and nanotechnology approach (Kang et al., 2003). In mechanical methods, the absorption rate is improved by modifying the shape, surface, and structure of the system (Isshiki et al., 1996; Kim et al., 2003). Chemical methods consist of adding surfactants to the base fluid (Kim et al., 2005), while in the nanotechnology approach; the mass transfer is enhanced by addition of nanoparticles.
The use of nanoparticles as additives in the absorption gas to improve the mass transfer fluid has attracted many researchers. Kim et al. carried out a study to investigate the effect of binary nanofluids on the absorption of gas in a bubble column. It was observed that the size of the bubbles generated in nanofluids is smaller than that in the base fluid; therefore, their absorption in nanofluids is fast. It is found that the absorption rate in nanofluids is greater than that in the base fluid and the absorption rate increases with increasing the concentration of nanoparticles. The results have shown that the absorption rate increased to 3.21 times by adding the nanoparticle (Kim et al., 2006) and this increase can be up to 5.32 times when surfactant and nanoparticle are used together (Kim et al., 2007). Kim et al. (Kim et al., 2008) added silica nanoparticle to water (base fluid) to absorb CO2 gas in a bubble column and observed that the absorption rate increases up to 76% during the first minute and total amount of absorption up to 24%. The effect of carbon nanotube on gas absorption is reported by Ma et al. (Ma et al., 2009). They concluded that addition of nanoparticles enhances mass transfer. Yang et al. (Yang et al., 2011) studied the effect of nanoparticles of Al2 O3, Fe2 O3, and ZnFe2 O4 in a binary nanofluid of ammonia-water on gas absorption in a wetted- wall column. They obtained the optimal values for each of nanoparticles and surfactants. For Fe2O3 and ZnFe2 O4 nanoparticles, the absorption rate increased 70% and 50% at 15% of the mass concentration of ammonia, respectively. Lee et al. (Lee et al., 2011) studied the effect of Al2O3 and SiO2 nanoparticles on CO2 absorption in a bubble column with methanol- based fluids. They found that there are optimal values for nanoparticles and the maximum absorption of CO2 for Al2O3 and SiO2 nanoparticles (compared to the pure methanol) are 4.5% and 5.6% at 0.01 vol%, respectively. Pineda et al. reported CO2 capture in a tray column with Al2O3 and SiO2 nanoparticles using methanol-based fluids (Pineda et al., 2012). They showed that maximum enhancement in the absorption rate (compared to the base fluid) are 9.4% at 0.05 vol% of Al2O3 and 9.7% at 0.05 vol% of SiO2. Jung et al. studied the effect of Al2O3 nanoparticle in methanol (base fluid) on absorption (Yung et al., 2012) and they found that the absorption rate of nanofluid is 8.3% (at 0.005 vol%) higher than the base fluid. The Effect of magnetic nanoparticle was studied by Wu et al. (2013) and Komati et al. (2008). They reported that addition of magnetic nanoparticles increase the absorption rate.
The effect of magnetic nanoparticles NiO and Fe3O4 and water as base fluid on CO2 absorption in a packed was reported by Salimi et al. (2015). They found that addition of nanoparticles into the solvent enhances mass transfer characteristics and the magnetic field showed positive effect on the CO2 absorption performance.
In the current study, the effect of nanofluids water/Al2 O3 and water/Al2 O3 - SiO2 mixture as a solvent has been investigated on CO2 absorption in a packed column. The concentration of nanofluids and the type of mixture nanoparticle are considered as key parameters influencing the absorption rate. Moreover, for the first time, the effect of mixing the nanoparticles on the absorption is investigated. The aim of the present work is to obtain optimal concentration, at which the maximum absorption rate occurs for nanoparticles.
2 Preparation of nanofluids
Preparation of nanofluids is the first step in the application of this concept. Nanofluids with more stability have more effective in enhancing mass transfer. Because some of the specific characteristics of nanofluid, such as high heat and mass transfer coefficient and the microconvection, have more effects in nanofluid with more stability than poor (Yang et al., 2011). In this research, a two-step method was used to prepare nanofluids. At first, Al2O3 (dp=15-20 nm) and SiO2 (dp=10-15 nm) nanoparticles were used at the concentrations of 0.005, 0.01, 0.05, 0.1 and 0.2%vol. Then, ultrasonic and mechanical stirrers were used to stabilize nanoparticles in the fluid. Finally, the solution was placed under ultrasonic oscillations for one hour. In order to avoid the deposition of nanoparticles in the base fluid, the prepared nanofluid immediately was used to the absorption CO2.
3 Experimental apparatus
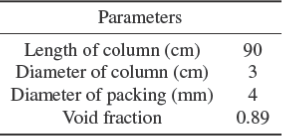
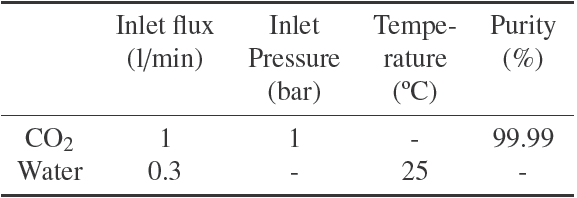
Fig. 1 shows the schematic view of the apparatus used to measure the gas absorption. The column was made of glass and was filled with Raschig ring packing. A round Lexan flow meter was used to measure the gas flow rate. GR flow meter was used to measure the liquid flow rate. Two thermometers (accuracy ±1 oC) were used at the outlet and inlet of the liquid to measure the liquid temperature. Glass wool was used to insulate the glass column and the operating conditions kept near isothermal conditions. The device properties and operational inlet conditions are shown in Tables 1 and 2, respectively.
CO2 gas enters from the bottom of the instrument and the fluid enters from the top of the tower as shown in Fig. 1. After steady state condition is achieved, CO2 concentration in the liquid outlet is measured by the titration method. The mass transfer rate (NA ) and the mass transfer coefficients (KLa) are calculated by the following equations:
where Q is the fluid flow rate, S the tower cross section, Z the tower height, Ci the feed concentration, Co the outlet concentration, and C* is the saturation concentration.
For validation, the results of experiments obtained for the mass transfer coefficients in pure water were compared to a model proposed by Billet and Schultes (Billet et al., 1999). They have offered the model base on 3500 experiments in packed columns for 46 different systems. The model is based on following equations:
All the experimental data have errors. Even if the experimental design is highly accurate, the presence of random errors will affect the results. If
And if Y = ln
To obtain the experimental error for final results, the following equations will be determined.
EQ= flow rate error (l/m)
EV = volume error (m3)
ECo = concentration error (mol/m3)
EY = error of Y (-)
ENA = mass transfer rate error (mol/m2s)
EKla = mass transfer coefficient error (1/s)
According to equations 3 and 4, the average errors for mass transfer rate and mass transfer coefficient are about 2.6% and 4%, respectively.
4 Discussion and conclusions
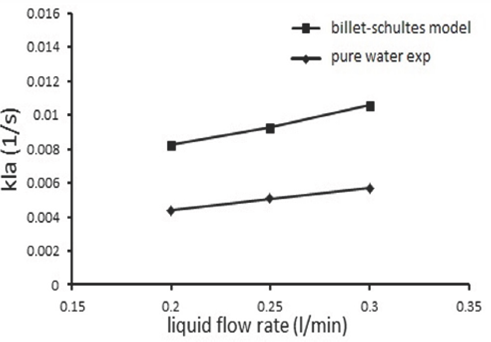
Fig. 2 shows a comparison between the experimental results obtained and Billet and Schultes model (Billet et al., 1999). It is clear that the trends are same and the differences might be due to the operating conditions and packing type.
Figs. 3 and 4 show the mass transfer rate based on the concentration of nanoparticles.
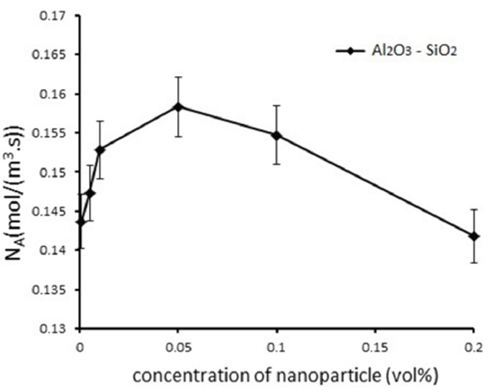
Fig. 4 Mass transfer rate at the diferent concentration of nanoparticles for mixture of Al2O3-SiO2 nanoparticles
It is found that the mass transfer rate increases with increasing the concentration of nanoparticles, and the maximum enhancement in the mass transfer rate is at 0.05 vol%. Therefore, the concentration of 0.05 vol% is an optimal value for CO2 absorption in nanofluids of water/Al2O3 and water/mixture of Al2O3 - SiO2. There is no mechanism that completely justifies the increased mass transfer rate with nanofluids; thus, such mass transfer enhancement is still speculative. The Brownian motion of nanoparticles cannot directly enhance the mass transfer (Krishnamurthy et al., 2006), but it is one of the main factors creating micro convection in nanofluids (Krishnamurthy et al., 2006; Kim et al., 2012; Sara et al., 2011). The grazing effect of nanoparticles is another parameter that increases the mass transfer (Kars et al., 2011; Kim et al., 2005). Due to this grazing effect, the gas molecules are adsorbed on the particles in the nanofluid and moved to the bulk liquid where they desorb.
Fig. 5 shows the effective mass transfer ratio (Ne f f ) obtained from following equation:
where Nnf and Nbf are the mass transfer rates of nanofluids and base fluids, respectively.
Fig. 5 shows that the maximum enhancement in the mass transfer rate for Al2O3 and mixture of Al2O3 - SiO2 are about 14% and 10% respectively, which occur at the concentration of 0.05 vol%. When the concentration of nanoparticles is greater than 0.05 vol%, the mass transfer rate will decrease. It can be due to decreased self-diffusion coefficient of the fluid (Pineda et al., 2012). Gerardi et al. (Gerardi et al., 2009) showed a reduction trend in the diffusion coefficient for Al2O3/ water and proposed two reasons for this phenomenon; first, the water molecules collide the nanoparticles and the curvature of their diffusion path are enhanced, second, the water molecules stick on the surface of particles and move with them, which have a diffusion coefficient smaller than the free molecules.
Another possible factor for reducing the mass transfer rate is that the nanoparticles at high concentrations stick together and they become larger and their Brownian motion is reduced (Kim et al., 2012; Lee et al., 2011). Also, viscosity increases with increasing the concentration of nanoparticles that is a negative factor in increased mass transfer rate (Yang et al., 2011).
Effects of nanoparticles volume fraction on mass transfer coefficient are shown in Figs. 6 and 7.
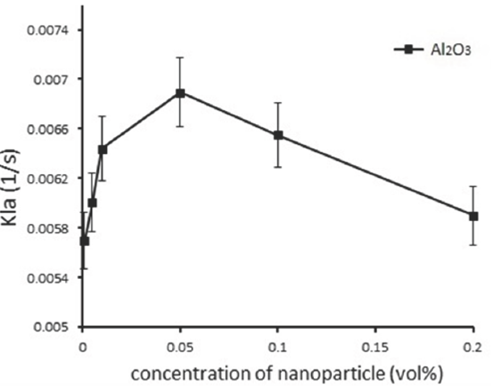
Fig. 6 Mass transfer coefficient at the different concentration of nanoparticles for Al2O3 nanoparticle.

Fig. 7 Mass transfer coefficient at the different concentration of nanoparticles for mixture of Al2O3- SiO2 nanoparticle
It is clear that the mass transfer coefficient increases with increasing the concentration of nanoparticles and the maximum enhancement in mass transfer coefficient is at 0.05 vol%. As noted previously, the mass transfer coefficient is a function of several factors such as the Brownian motion of nanoparticles, as well as diffusivity and viscosity of the fluid. It is found that beyond 0.05 vol%, reduced diffusion coefficient and increased viscosity are more effective than the micro convection created by the Brownian motion of nanoparticles; therefore, the mass transfer coefficient is reduced.
When the nanoparticle concentration is upper a critical concentration, the amount of nanoparticles become too dense, so that they have a negative effect and reduce the absorption process; therefore, the viscosity is increased highly with increasing nanoparticle concentration (Lee et al., 2015). On the other hand, the thickness of diffusion layer is directly proportional with the viscosity. Enhancement of the nanofluid viscosity increases the thickness of the diffusion boundary layer; furthermore, the mass transfer coefficient decreases (Syam Sundar et al., 2013, Samadi et al, 2014).
Fig. 8 shows the effective mass transfer coefficient (Ke f f ) obtained from equation 3:
where Knf and Kbf are the mass transfer coeffient of nanofluids and the mass transfer coeffient of base fluids, respectively.
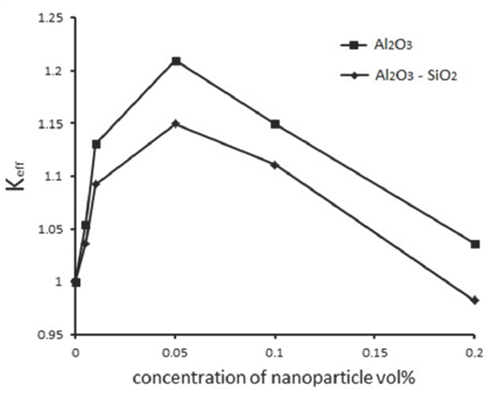
The maximum enhancement in mass transfer coefficient for Al2 O3 and mixture of Al2 O3 - SiO2 are about 20% and 15%, respectively, which occured at the concentration of 0.05 vol%. Fig 8 also shows a comparison between the nanoparticles of Al2O3 and mixture of Al2O3 - SiO2 in the CO2 absorption process that shows, Al2 O3 nanoparticles have performance better than that observed for combined Al2 O3 - SiO2 . This may be due to the different structure of Al2O3 and SiO2. Yang (Yang et al., 2010) studied the effect of α- Al2O3 and γ-Al2O3 nanoparticles on gas absorption and concluded that γ-Al2O3 has better performance than α-Al2 O3 . The two types of Al2 O3 have different structures because of different properties of their surface, which leads to different ability to increase the mass transfer rate.
Conclusions
In this study, the nanofluids of water/Al2O3 and water/mixture of Al2O3 - SiO2 were used for gas absorption in a packed column and the following results were obtained:
Both the mass transfer rate and the mass transfer coefficient increased with adding the nanoparticles to the base fluid.
The maximum enhancement in mass transfer and the mass transfer coefficient for Al2 O3 nanoparticle was around 14% and 20%, respectively, which occurred at the concentration of 0.05 vol%.
The maximum enhancement in mass transfer and the mass transfer coefficient for mixture of Al2O3-SiO2 nanoparticle was around 10% and 15%, respectively, which is related to the concentration of 0.05 vol%.
Nomenclature
N A |
mass transfer rate, mol s−1 m3 |
Q |
volume flow rate, m3 s−1 |
S |
cross-sectional area, m2 |
Z |
column length, m |
C |
concentration, mol m−3 |
Kla |
mass transfer coefficient, s−1 |
Y |
parameter |
E |
error |
α |
specific surface area of packing, m2m−3 |
C |
constant in Eq. 7 |
d |
diameter, m |
D |
diffusion coefficient, m2 s−1 |
Fr |
Froude number |
g |
gravitational acceleration, ms−2 |
h l |
column holdup, m3 m−3 |
Re |
Reynolds number |
Ū |
mean effective velocity, ms−1 |
U |
velocity, ms−1 |
We |
Weber number |
d h |
hydraulic diameter, m |
σ L |
surface tension, kgs−2 |











 nueva página del texto (beta)
nueva página del texto (beta)