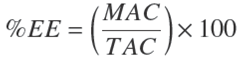1 Introduction
Curcumin is a polyphenol derived from the rhizome of the herb Curcuma longa. Traditionally, it has been used for many conditions, particularly as an anti-inflammatory agent (Gupta et al., 2012). Curcumin also possesses antioxidant, antiviral, antibacterial, antifungal, antiparasitic, antitumoral, and anticarcinogenic activities. Additionally, it is considered a hepato-, nephro-, and myocardial infarction- protector (Gupta et al., 2013). It has also been established that curcumin displays immunomodulatory and antirheumatic activities. These effects are mediated through the regulation of various transcription and growth factors, inflammatory cytokines, protein kinases, and other enzymes (Aggarwal et al., 2007).
The pharmacological safety and efficacy of curcumin makes it a potential compound for treatment and prevention of a wide variety of chronic illnesses, diabetes, allergies, arthritis, and Alzheimer's disease (Aggarwal and Sung, 2009). Similarly, the efficacy of curcumin in various malignant diseases including cancer has been established (Shanmugam et al., 2015). Nevertheless, the poor bioavailability of curcumin has been underlined as a major drawback. The first reported study that examined the uptake of curcumin using Sprague-Dawley rats showed negligible amounts of curcumin in the rat's blood plasma after oral administration of 1 g/kg of curcumin, suggesting that curcumin was poorly absorbed from the gut (Prasad et al., 2014). Recently, nanotechnological delivery systems for drugs and nutraceuticals have emerged as promising solutions to improve the bioavailability of therapeutic agents and bioactive compounds (Wang et al., 2014).
Nanoemulsions, fine oil-in-water dispersions stabilized with small amounts of emulsifiers, are small enough not to scatter light in the visible region of the spectra; thus, they appear clear instead of being optically opaque. Compared to micron-sized emulsions, nanoemulsions provide a greater surface area and have the potential to increase solubility due to a combination of large interfacial adsorption of the core compound, enhance the bioavailability by ensuring rapid delivery of active ingredients to cell membranes, and improve the controlled release, all of which enable better precision targeting of the encapsulated materials (Lovelyn and Attama, 2011). Furthermore, because of their small size, nanoemulsions do not cream after relatively long periods of storage (Hernández-Jaimes et al., 2013).
Different processes have been used to obtain nanoemulsions although, the most widely used for the preparation of oil-in-water nanoemulsions comprise high-energy emulsification methods such as high-pressure homogenization, microfluidization, and ultrasonication, all of which can reduce the globule size to nano-scale (Monroy-Villagrana et al., 2014; Silva et al., 2012). Compared with the traditional mechanical methods, ultrasound is a superior tool to prepare nanoemulsions with smaller and homogeneous globule size and physical stability; and the energy consumption is considerably lower than other classical devices (Tang et al., 2013).
The purpose of the present work was to evaluate the effect of varying the composition and preparation method on the entrapment efficiency, curcumin concentration, droplet size, and polydispersity index of curcumin nanoemulsions prepared by ultrasonication and stabilized by soybean lecithin.
2 Materials and methods
2.1 Chemicals and reagents
Curcumin (C, purity ≥ 98 %) was purchased from LKT Laboratories (St. Paul, MN) and soybean lecithin (SL, 95 % phosphatidylcholine) from Avanti Polar Lipids (Alabaster, AL). Medium chain oil (MCO) composed of caproic acid-C6:0 (1.65 mol %), caprylic acid-C8:0 (67.14 mol %), C10:0-capric acid (30.57 mol %), and lauric acid-C12:0 (0.64 mol %) was used as oil phase solvent (Original Thin Oil®, Sound Nutrition, Dover, ID) and glycerol (Gly; 99.5 % USP grade, vegetable-based, KIC Chemicals, Inc., New Paltz, NY) as aqueous phase co-solvent. HPLC grade solvents were purchased from Tecsiquim (Mexico City) and all other reagents were purchased from SIGMA (Mexico City). Deionized distilled water (DW) was used in all the experiments.yellow, red, purple and black colors grown in Querétaro, México.
2.2 Preparation of nanoemulsions by ultrasonication
Emulsions with MCO as the dispersed phase (5 %) and DW as continuous phase (85 %), with SL as emulsifier (10 %), were prepared to evaluate the effect of the ultrasonic operating parameters as features of the device, ultrasonic amplitude, and irradiation time, used in its preparation. The emulsions were prepared adding the dispersed phase to the continuous phase and this mixture was pre-emulsified for 3 min at 20,000 rpm using a T25 digital ULTRA-TURRAX homogenizer (IKA Works, Inc., Wilmington, NC). The formed coarse emulsion was passed through the sonication devices: Branson Digital Sonifier S-450D (Emerson Electric Co., St. Louis, MO) or an Ultrasonic Processor VCX 130 PB (Sonics & Materials, Inc., Newtown, CT.) at determined ultrasonic amplitude (10-80) and irradiation time (1-15 min) in order to evaluate the effects on droplet size (DS) and polydispersity index (PDI) of the obtained nanoemulsions.
2.3 Preparation of curcumin nanoemulsions
Initially eight curcumin nanoemulsion (CN) formulations were produced by combined 14 evaporation-emulsification and ultrasonication 15 methods (EES) described by Ganta and Amiji (2009) with slight modifications, varying ingredients composition as indicated in Table 1. Briefly, the continuous phase was prepared by adding SL to pure DW, or a mixture (1:1) of DW and Gly (45, 42.5, 40, or 37.5 % w/w NE), and stirred for 15 min. The oil phase was prepared adding C previously solubilized in absolute ethanol to MCO, and the solvent was evaporated using a nitrogen stream. Both the aqueous and the oil phases were heated by separate at 75 °C for 4 min. The oil phase was dispersed drop-wise into the continuous aqueous phase with stirring. After the last oil phase drop was added, the mixture was homogenized for 3 min at 20,000 rpm using a T25 digital ULTRA-TURRAX homogenizer to produce a coarse curcumin-in-water emulsion. The coarse emulsion was then ultrasonicated at 20 % amplitude using a Branson Digital Sonifier S-450D for 12 min to obtain the nano-sized droplets; the probe diameter used was 13 mm.
Table 1 Composition of curcumin nanoemulsion formulations produced by the evaporation-emulsification and ultrasonication methods (EES)
These formulations were made with MCO 5 % w/w and C (2.5 mg/g NE).
Then, the formulation that displayed the smallest mean droplet size and had a translucent appearance was selected, and used as the starting point to prepare ten more CN varying the curcumin load as indicated in Table 2, by thin-film hydration emulsification and ultrasonication methods (TFHES) as reported by Anuchapreeda et al. (2012) with slight modifications. Briefly, the SL was dissolved in 5 mL of absolute ethanol by stirring for 5 min and then added to MCO, with further stirring for 3 min. C was added to the mixture, dispersed by vigorous mixing and sonicated for 15 min in a Barnstead Aquawave 9376 ultrasonic bath (1.75 L, 115 V, 50/60 HZ, 500 W). The mixture was rotary evaporated to generate the dried thin film, which was hydrated with pure DW (85 % w/w NE), or a mixture of DW + Gly (42.5 + 42.5 % w/w NE), warmed at 45°C and stirred for 15 min. The mixture was homogenized as describe2d above to produce the coarse curcumin-in-water emulsion, which was subsequently ultrasonicated under the same conditions described above in order to obtain the nano-sized droplets.
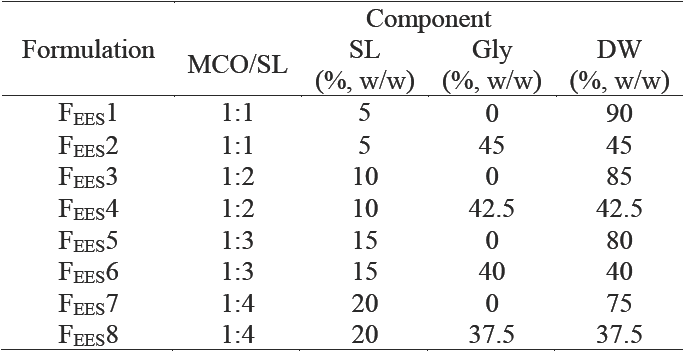
Table 2 Composition of curcumin nanoemulsion formulations produced by the thin-film hydration-emulsification and ultrasonication methods (TFHES)
These formulations were made with MCO 5 % w/w and SL 10 % w/w (ratio MCO/SL 1:2).
All nanoemulsions were prepared in duplicate, and evaluated for droplet size (DS), polydispersity index (PDI), zeta potential (ζ), curcumin concentration (CC), and entrapment efficiency (EE).
2.4 Characterization of CN
The DS and PDI of CN were measured with a Nano-ZS90 dynamic light scattering device (Malvern Instruments Inc., Worcestershire, UK) at 90° fixed angle at 25 °C. DS were reported as "Z-average" diameters (the scattering intensity-weighted mean diameter), which were calculated from the signal intensity versus DS data. Samples were diluted with DW prior to making the DS measurements to avoid multiple scattering effects, using a 1:200 sample-to- water dilution factor. For the ζ, CN samples were diluted with DW using a dilution factor of 1:100 sample-to-water, placed in the electrophoretic cell of the Zetasizer, and the average surface charge was determined.
2.5 Determination of CC and EE
CC in CN was determined by HPLC according to the method reported by Shaikh et al. (2009). A standard curve was prepared from known concentrations of C in ethanol. The CN was centrifuged at 4,000 rpm (2,057g) at 5 °C for 10 min using a centrifuge (Eppendorf 5810R, rotor radius: 11.5 cm). The supernatant was filtered through a membrane filter to remove the remaining insoluble curcumin; 5 mL of ethanol were added to 5 μL of the filtering supernatant CN and 10 μL were injected into a Waters HPLC System fitted with a Waters Econosphere C18 (5 μm, 250 X 4.6 mm) column. C was detected at 428 nm with a Waters UV-visible detector model 2487. The mobile phase consisted of acetonitrile, 2.8% acetic acid and methanol, at a flow rate of 1 mL/min. Retention time for C was 4.6 min.
The amount of C in the CN was calculated from the final concentration of C after preparation and centrifugation. Curcumin EE in the nano-emulsion was calculated with the following equation:
Where: %EE is the entrapment efficiency of curcumin in the CN, in percent; MAC is the measured amount of curcumin in CN, in mg/g NE; and TAC is the total amount of curcumin used to prepare CN, in mg/g NE.
3 Results and discussion
3.1 Impact of ultrasonic operating parameters on nanoemulsions
Nanoemulsions can be prepared by using the chemical potential of the components (low-energy emulsification), or by means of a mechanical device (high-energy emulsification); high-energy methods used for preparation of nanoemulsions include ultrasonication, which can reduce the globule size at nanometric scale (McClements, 2010; Solans et al., 2005). However, it is necessary to evaluate the possible operating and compositional variables during the formation of nanoemulsions by ultrasonication. In this study, nanoemulsions prepared with MCO as the dispersed phase, DW as continuous phase, SL as emulsifier, and previously pre-emulsified in the homogenizer, were passed through the sonication devices (Branson Digital Sonifier S-450D or Ultrasonic Processor VCX 130 PB) to an irradiation time of 1 min, to different ultrasonic amplitude (10-80 %), to examine the dependence of emulsion droplet size and polydispersity index on this operating variable. The results are presented in Figure 1. Irrespective of the device used, the ultrasonic amplitude had a significant effect on the droplet size (ANOVA F = 59.92; p < 0.001) and polydispersity index (ANOVA F = 71.20; p < 0.001) of the nanoemulsions produced. When the ultrasonic amplitude increased from 10 to 30 % no significant differences (p < 0.05) in the mean droplet size of the nanoemulsions were found. However, at greater ultrasonic amplitude (40-80 %) the mean droplet size increased (p < 0.05). The polydispersity index was found significantly higher for the ultrasonic amplitudes of 70 and 80 %.
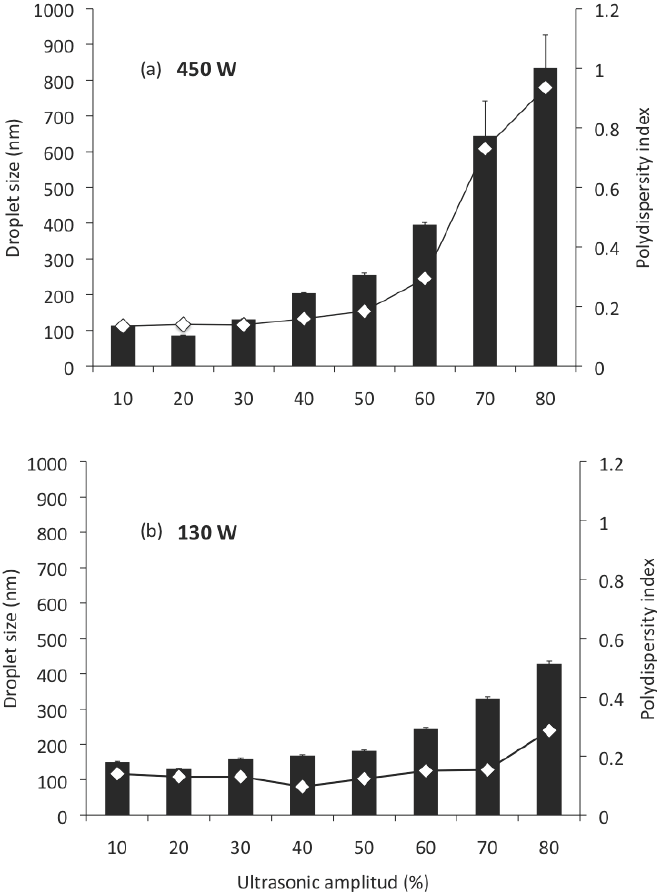
Figure 1 Droplet size and polydispersity index of nanoemulsions prepared by sonication at an irradiation time of 1 min, at different ultrasonic amplitudes; (a) corresponds to the Branson Digital Sonifier S-450D and (b) to the Ultrasonic Processor VCX 130 PB. Nano-emulsions were prepared with 85 % of DW, 5 % of MCO, 10 % of SL.
According to Leong et al. (2009), the intense shear force applied during the formation of an emulsion depends on the energy input, which is the product of power delivered and the residence time. The variation in ultrasonic amplitude from 10 to 80 % represented an increase in the energy delivered to the emulsion. But nevertheless, an excess energy supply causes an intense turbulence promoting a higher rate of collision between droplets, their coalescence and larger droplet sizes (Jafari et al., 2006). This phenomenon, where the particle size increased with increased input energy, referred as "over-processing", could be attributed to poorer function of the emulsifiers, and an increase in the Brownian motion; hence, to higher probability of collision and coalescence at higher energy input. In these conditions, the droplet size distribution of the emulsion is a result of the competition between two opposite processes, drop breakage and drop-drop coalescence (Tang et al., 2012).
Fresh interface is created whenever a droplet is formed from breakage of an original droplet. Between its formation and its subsequent encounter with the other droplets, some surfactant will adsorb onto this fresh interface. If the timescale of collision is shorter than the timescale of adsorption, the fresh interface of the newly formed droplets will not be fully covered with surfactant and leads to coalescence. So, drop coalescence will depend on the relative rates of surfactant adsorption and drop collision. In our case, at higher ultrasonic amplitudes, the rate of drop collision is greater than drop breakage. It represents a greater coalescence rate, leading to an increase in the droplet size.
Also, similar nanoemulsions prepared with MCO, DW, and SL were passed through the sonication devices, to an ultrasonic amplitude of 20 %, to different irradiation times from 1 to 15 min; At this ultrasonic amplitude, the irradiation time had a significant effect on the size (ANOVA F = 63.41; p < 0.001) of the droplets produced, and on the polydispersity index (ANOVA F = 6.41; p < 0.01) of the nanoemulsions. Increasing irradiation time from 1 to 10 min the mean globule size was significantly reduced (p < 0.05); however, after 10 min, no significant decrease in mean globule size was observed (Figure 2). In this sense, Cavazos-Garduño et al. (2014) studied the effects of ultrasonic amplitude, irradiation time, and emulsifier concentration in the characteristics of betulinic acid nanoemulsions. They found that the globule size was affected by ultrasonication conditions, mainly by the irradiation time; from 20 to 40 % of ultrasonic amplitude. When the irradiation time increased the mean globule size was reduced. However, after 250 s no further decrease in mean globule size was observed.
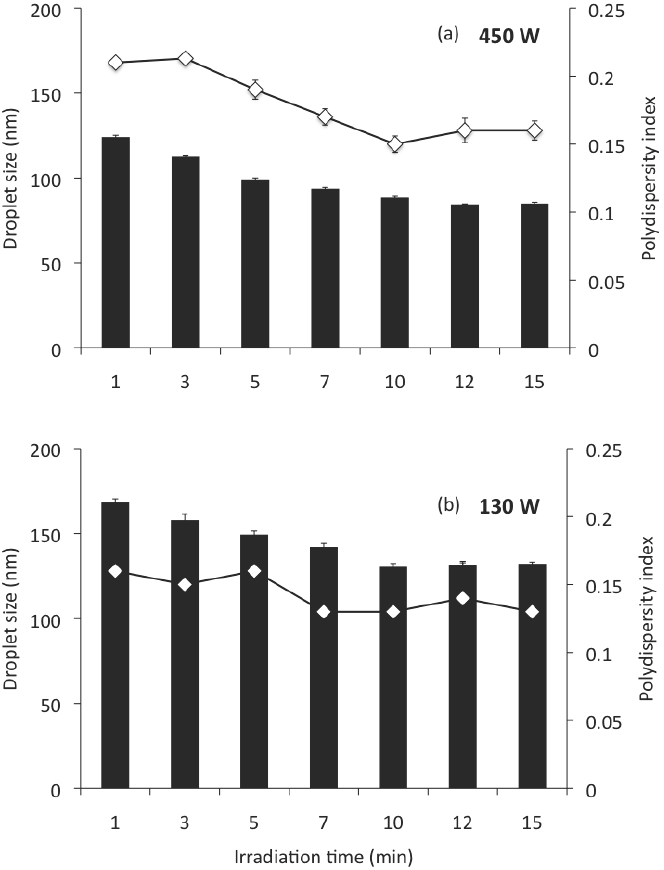
Figure 2 Droplet size and polydispersity index of nano-emulsions prepared by sonication at an ultrasonic amplitude of 20 %, at different irradiation times; (a) corresponds to the Branson Digital Sonifier S 450D and (b) to the Ultrasonic Processor VCX 130 PB. Nano-emulsions were prepared with 85 % of DW, 5 % of MCO, 10 % of SL.
According to Leong et al. (2009), the minimum droplet size and the time required to achieve the minimum droplet size is clearly affected by the equipment configuration used. In this work, the equipment used had significant effect on the droplet size (ANOVA F = 14.09; p < 0.001) and the polydispersity index (ANOVA F = 11.52; p < 0.01) of the nanoemulsions produced. The effect of the sonication device used on the preparation of nanoemulsions on droplet size and polydispersity index can be examined in Figures 1 and 2; in these, (a) corresponds to the Branson Digital Sonifier S- 450D and (b) to the Ultrasonic Processor VCX 130 PB, at a determined ultrasonic amplitude (Figure 1) and a determined irradiation time (Figure 2). At the same conditions of ultrasonic amplitude (Figure 1), the effect of the sonication device on globule size showed, in applying sonication amplitudes of 10, 20 and 30, a smaller globule size (p < 0.05) for the sonication device (a), which could be expected because of their greater power of sonication; however, at higher sonication amplitudes (40-80), the globule size was much higher (p < 0.05) for this sonication device than for sonication device (b). This can be explained, as previously mentioned for over processing, which is expressed more on the sonication device with a higher power. Similarly the polydispersity index was found to be much higher (p < 0.05) for the device of sonication power of 450 W (up to 0.94) than for the device of sonication power of 130 W (0.14-0.29). In all cases, smaller globule sizes (p < 0.05) can be noted for the device (a) at the same conditions of irradiation time, compared with the device (b) (Figure 2). Although the polydispersity index was found to be higher (0.21 - 0.16) than for the sonication device of 130 W of power sonication (0.16 - 0.13).
3.2 Impact of SL concentration on CN
The mean droplet size of nanoemulsions are a function of the formulation composition and preparation conditions (Hernández-Jaimes et al., 2013; Chaparro- Mercado et al., 2012), so that droplet size may be tailored by formulation design in order to attain the desired stability, physicochemical properties and functional performance (McClements, 2010). Nanoemulsion characteristics were affected by composition, independently of the preparation method employed. Increasing SL for a constant C loading (2.5 mg/g NE), Formulations in Table 1, had a significant effect (ANOVA F = 7,485; P < 0.001) on the size of the droplets produced (Figure 3a). At 10 % w/w of SL on NE, which corresponds to a 1:2 ratio of oil-to- emulsifier, the mean droplet diameter produced was 113 nm, while the mean droplet diameter produced with less emulsifier (5 % w/w on CN) was greater (P < 0.05) than this value (ca. 140 nm), which suggested that there was insufficient emulsifier to cover all the droplets formed by ultrasonication. For these SL concentrations there was a decrease in mean droplet diameter with increasing emulsifier concentration. This trend was expected because there should be more emulsifier present to cover any new droplet surfaces formed during ultrasonication, and because the droplet surface is more rapidly covered by a layer of emulsifier molecules. Nevertheless, higher increases of SL concentration (15 and 20 % w/w on CN) caused an increase (P < 0.05) in globule size (142 and 164 nm, respectively). This could occur through a number of mechanisms: (a) multilayers may have formed around each droplet; (b) droplet flocculation may have been promoted; or (c) LS aggregates may have been formed in the continuous phase that contributed to the light scattering signal (Qian and McClements, 2011).
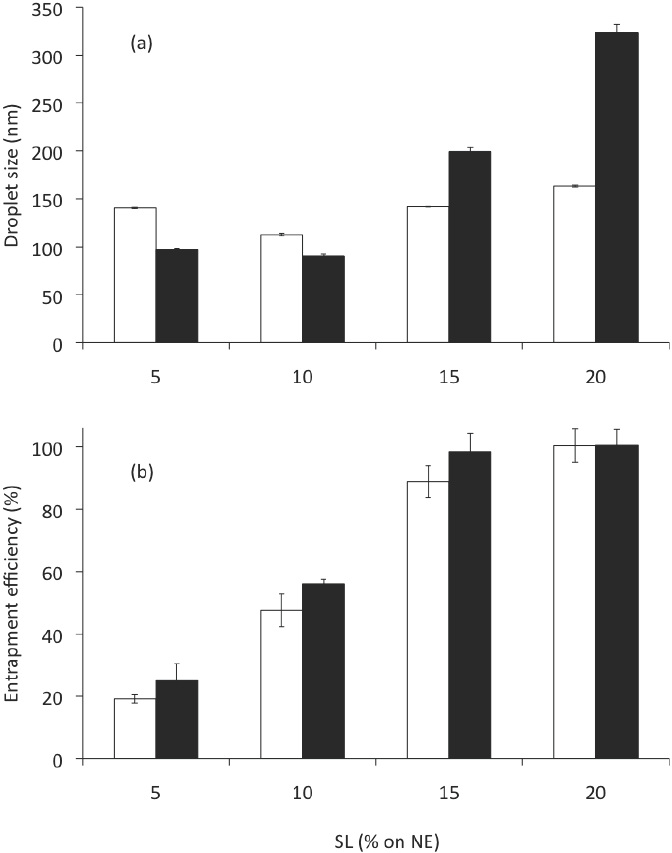
Figure 3 Gly and SL effect on (a) the droplet size and (b) the entrapment efficiency of CN, corresponding to formulations in Table 1. Open bar: formulations without glycerol. Solid bar: formulations with glycerol. Preparation method: Evaporation-emulsification and ultrasonication. Data are represented as the mean ± standard deviation of 2 independent experiments (n = 2).
PDI values are related to the quality of the dispersion and represent the width of the size distribution. PDI values smaller than 0.1 or 0.2 indicate suitable measurements and overall good quality of the colloid suspension. Values close to 1 are found for poor quality emulsions, which either do not represent droplet sizes in the colloidal range or exhibit a very high polydispersity (Klang and Valenta, 2011). In this work, the SL also had significant effect on the polydispersity index (ANOVA F = 85.81; p < 0.001) of the CN. The PDI of the CN produced (0.13, 0.14, 0.18 and 0.22 to 5, 10,15 and 20% w/w of SL, respectively) also caused that elevated concentrations of LS increase (P < 0.05) the dispersion and the width of the size distribution.
When CN were examined for entrapment efficiency (% EE), after centrifugation at 2,057g for 10 min, we found that % EE changed significantly (ANOVA F = 108.59; p < 0.001) depending on the amount of emulsifier used (Figure 3b). At 0.5 g of SL on NE (5 % w/w of emulsifier), the % EE was only 19; this value increased significantly (P < 0.05) to 100 when 2 g of emulsifier were employed (20 % w/w), which suggests a high affinity of curcumin for LS. Nonetheless, higher increases on LS concentration caused an increase in mean droplet size and PDI. Moreover, in practice it is often desirable to minimize the total amount of emulsifier employed to prepare an emulsion due to economic, sensory, or regulatory reasons.
3.3 Impact of Gly on CN
The addition of Gly in the aqueous phase of CN prepared by the EES (see Formulations in Table 1) showed a significant effect on the droplet size (ANOVA F = 2,872; p < 0.001) and the polydispersity index (ANOVA F = 21.68; p < 0.001) of the nanoemulsions. Its addition resulted in all cases in significant changes in mean droplet size (see solid bars in Figure 3a). But, while the mean droplet sizes were significantly smaller (98 and 90 nm) at the lower concentrations of SL as emulsifier (5 and 10 % w/w on CN) than the same formulations prepared without Gly (open bars in Figure 3a); mean droplet diameters prepared with more LS added (15 or 20 % w/w) produced significantly higher mean droplet diameters (199 and 323 nm, respectively) than the same formulations without Gly. A possible explanation of the droplet size reduction by addition of Gly at low LS concentrations is that Gly in the continuous aqueous phase reduced water's surface tension. LS moved freely and was able to absorb around the MCO droplet, thereby resulting in an increased surface to volume ratio of the droplet (Zhou et al., 2010). Increased SL concentrations resulted in a sharp increase in the viscosity of the continuous aqueous phase. This higher viscosity generated difficulty in SL movement and a slower diffusion and further adsorption at the oil droplet during the preparation procedure. According to McClements (2012), changes in viscosity of the continuous phase may influence droplet size through a number of different mechanisms: (a) increased droplet fragmentation produced by increased disruptive shear stresses; (b) decreased droplet re-coalescence due to decreased droplet collision frequency; or (c) increased droplet re-coalescence caused by a reduction in emulsifier adsorption rate. However, the relative importance of these factors is highly system-dependent, and varies with the type of mechanical device employed and the operating conditions (Hakansson et al., 2009).
Irrespective from the mean droplet size, an effect of Gly on the NE appearance was also observed. When the formulation was composed of 5 and 10 % w/w of SL, the NE appeared as an opaque solution. However, when the formulation was prepared with Gly as co-solvent, the NE appeared as a semi-transparent solution; as it was previously reported by Zhou et al. (2010).
In contrast to the concentration of emulsifier present on CN preparation, addition of Gly, at all SL concentrations tested, did not affect the % EE. NE prepared with or without Gly achieved similar % EE (Figure 3b).
3.4 Impact of the Method of Preparation on CN
CN prepared at 10 % w/w SL (1:2 ratio of oil to SL), 42.5 % Gly, and 2.5 mg of curcumin loading per g NE by the EES method (Formulation FEES4, in Table 1), was considered according to the impact of SL concentration and addition of Gly on CN, as the best formulation with the smallest droplet size (DS) and polydispersity index (PDI); it showed a pellet of curcumin at the bottom after been centrifuged in order to separate the excess curcumin.
The EES method produced CN with only 56 % EE (Table 3). In this sense, many hydrophobic bioactive compounds like curcumin are crystalline at ambient temperature and are often difficult to incorporate into fluid products because of their tendency to aggregate and sediment (Porter and Wasan, 2008). These crystalline hydrophobic bioactive components may be dispersed within a carrier lipid prior to emulsion formation, but it is important to ensure that the entire lipid phase remains liquid throughout the process, which can be accomplished using two different approaches: melting and dissolution. In practice, the first approach is unsuitable for many hydrophobic bioactive components because they may be chemically unstable at elevated temperatures, so they are often dissolved in a solvent. This solvent may be a carrier lipid (such as a triacylglycerol oil) or an organic solvent (such as an alcohol) but, from a thermodynamic point of view, a crystalline material has a finite solubility in a given solvent that depends on the nature of the bioactive component, the nature of the solvent, and environmental conditions. It is also possible to use a combination of dissolution and melting steps to produce emulsions containing encapsulated hydrophobic components. In this case the crystalline material is dispersed in the carrier oil, which is then heated to dissolve the crystals. An oil- in-water emulsion is then formed by homogenization of the liquid oil and water phases together in the presence of a water-soluble emulsifier. The advantage of this method is that considerably lower temperatures can be used to melt all of the crystalline material. Nevertheless, according to Li et al. (2012) crystals may form in the emulsion when the system is cooled and then they may aggregate and sediment.
Table 3 Effect of preparation method on several nanoemulsion characteristics
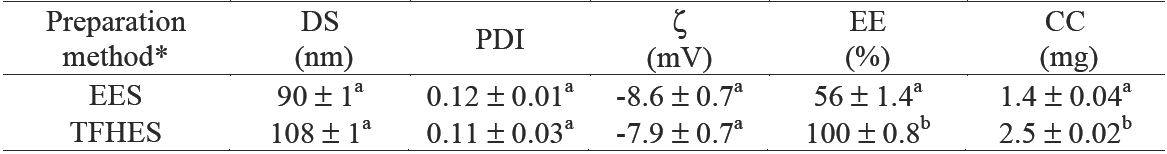
EES = evaporation-emulsification and ultrasonication methods; TFHES = thin-film hydration-emulsification 22 methods DS = droplet size; PDI = polydispersity index; ζ = zeta potential; EE = entrapment efficiency; CC = curcumin concentration. Values reported are mean ± SD. n=2. Means with different letters are significantly different (P < 0.05) by one-way ANOVA.
* Corresponding to formulations FEES4 in Table 1 and FTFHES4 in Table 2.
In order to achieve greater % EE, CN with the same formulation (10 % w/w SL, 1:2 ratio of oil to SL, 42.5 % Gly, and 25 mg of curcumin loading) were also prepared by the TFHES (Formulation FTFHES 4, in Table 2). The method of preparation of CN showed a significant effect on the entrapment efficiency (ANOVA F = 14.09; p < 0.001) of the nanoemulsions. This method of preparation of CN showed significantly better results of entrapment efficiency (100%), in contrast with the EES method (56%), which corresponds to 2.5 and 1.4 mg of curcumin entrapped/g NE, respectively. Other variables as mean droplet size and distribution, and surface charge had no significant effects (Table 3). According to McClements (2012), a variety of substances have shown to inhibit nucleation and thereby promote supersaturation. Incorporation of these substances into a system may be useful for preventing undesirable nucleation and crystal growth of a bioactive component. These substances include additives capable of forming micelles or vesicles such as phospholipids that may be able to incorporate hydrophobic solutes within the non-polar regions of their internal structures. Consequently, the maximum concentration of solute that can be solubilized in the system would be increased, leading to a reduction in the driving force for nucleation. Whether the CN is prepared by TFHES the SL form part of the lipid phase, this is dissolved in ethanol together with MCO and then C is added and dissolved in the mixture. The lipid phase is dried to evaporate the solvent and then the aqueous phase (DW or DW and Gly) is added to generate an emulsion. In this way the presence of SL in the lipid phase increases or promotes the solubility of curcumin and allows greater entrapment efficiency.
3.5 Impact of C loading on CN
Because the main goal of this study was to develop a formulation of a CN with the highest C loading, CN with were prepared by the TFHES. The 10 formulations (at 10 % of SL) are listed in Table 2. The C loading on CN showed a significant effect on the droplet size (ANOVA F = 150.54; p < 0.001), polydispersity index (ANOVA F = 101.09; p < 0.001), zeta potential (ANOVA F = 183.85; p < 0.001), and entrapment efficiency (ANOVA F = 10,205; p < 0.001) of the nanoemulsions prepared. The effects of the C loading on CN on the mean droplet diameters produced are shown in Figure 4a. As the amount of C applied increased from 0 mg/g NE (F TFHEs 2) to 2.5 mg (FTFHEs4), 5.0 mg (F TFHEs 6), 7.5 mg (F TFHEs 8), and 10.0 mg (F TFHEs 10), the mean diameter of the NE increased significantly (p < 0.05) from 77 to 108, 156, 186, and 243 nm, respectively. The PDI varied from 0.12 to 0.11, 0.48, 0.51, and 0.59.
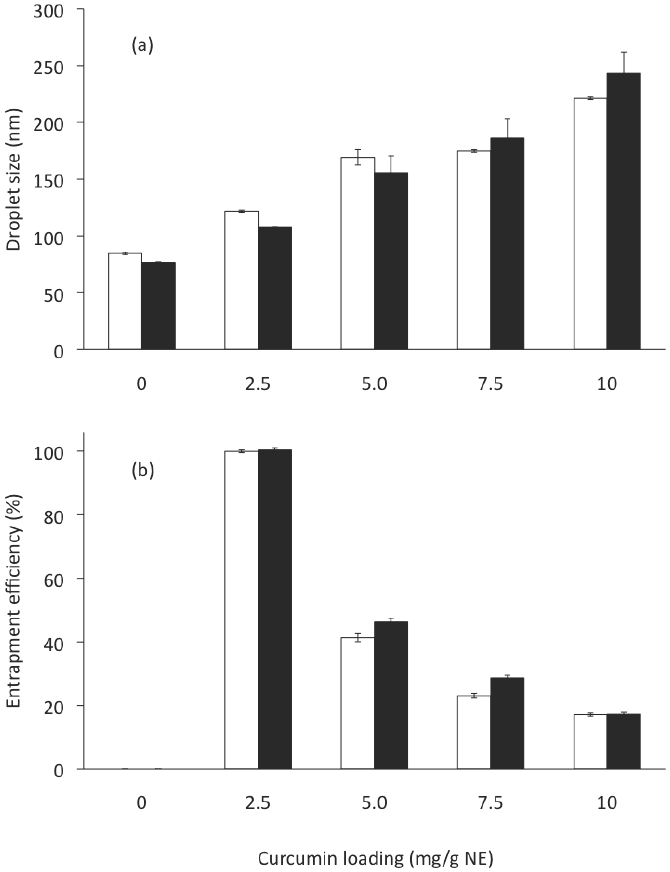
Figure 4 Curcumin loading and Gly effect on (a) droplet size and (b) entrapment efficiency of CN, corresponding to formulatio8ns in Table 2. Open bar: formulations without glycerol. Solid bar: formulations with glycerol. Preparation method: Thin-film hydration-emulsification and ultrasonication. Data represent the mean ± standard deviation of 2 independent experiments (n = 2).
CN were examined for entrapment efficiency (% EE). When CN were centrifuged to separate the non- emulsified C after sonication, pellets of excess C could be observed when more than 2.5 mg of C were added. We found that the % EE decreased significantly (p < 0.05) by increasing the total amount of C applied (Figure 4b), thereby implying that there were excess amounts of free C after the emulsification process. From 25 to 50, 75, and 100 mg of C, the % EE in the NE were: 100, 46, 29, and 17, respectively. These results are consistent with those reported by Anuchepreeda et al. (2012). Amounts of C entrapped in the CN were 2.5, 2.3, 2.1 and 1.7 mg per g of NE, to 2.5, 5.0, 7.5 and 10 mg of C applied per g of NE. These results indicate that for this CN formulation (10 % w/w SL, 5 % w/w MCO, 42.5 % w/w Gly, and 42.5 % DW), its maximum ability to incorporate C was 2.5 mg per g NE. Compared to the loading amount of C in NE in a previous report (Anuchapreeda et al., 2012), our formulation exhibited a 3-fold greater loading of C, while other reports indicate amounts of C entrapped from 0.25 to 2 mg per g (Ahmed et al., 2012; Chen et al., 2011; Ganta and Amiji, 2009; Li et al., 2014; Sari et al., 2015).
3.6 Stability of CN
The time course-dependent change in the dispersion and mean droplet diameter of the CN, prepared with 2.5 mg of C/g NE and 42.5 % w/w of Gly, were evaluated during storage at 4°C for 180 days are presented in Table 4. The mean droplet diameter changed from 108 to 169 nm at day 120, while no significant changes (p < 0.05) on polydispersion (PDI) were observed. The zeta potential of CN ranged from -7.9 to -12.3. Based on the zeta potential data, it is likely than an electrostatic repulsion between the nanoemulsion droplets played a role in slowing down droplet flocculation, and contributed to their long-term stability. Although only particles with zeta potential values higher than +30 mV or lower than - 30 mV are generally considered to be stable due to the strong repulsion forces (Liu et al., 2012), apart from charge-induced stabilization, phospholipids also lead to improve molecular packing at the interface, and nanoemulsion stability. Phospholipids with short and saturated acyl hydrocarbon chains decrease the molecular packing and promote the formation of stable nanoemulsions (Klang and Valenta, 2011).
Table 4 Stability characteristics of the best formulation obtained by thin-film hydration emulsification and ultrasonication methods (TFHES)
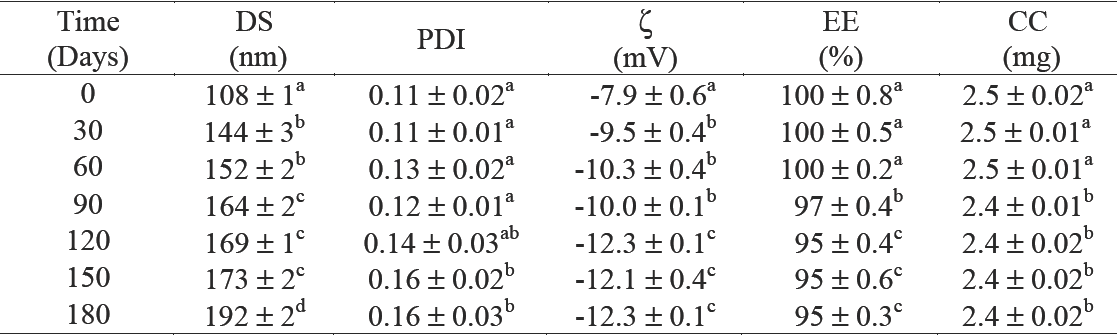
DS = droplet size; PDI = polydispersity index; ζ= zeta potential; EE = entrapment efficiency; CC = curcumin 32 concentration. Values reported are mean ± SD. n=2. Means with different letters are significantly different (P < 33 0.05) by one-way ANOVA. 3345
*Corresponding to formulation FTFHES4 in Table 2.
On the entrapment efficiency (% EE), we found that % EE decreased during storage from 100 to 95 after 180 days (Table 4). This represents a reduction of only 5 % EE. Nevertheless, when Gly was not used as co-solvent, the CN showed a significantly (p < 0.05) higher mean droplet diameter during storage (see Figure 5a) and a significantly higher reduction on % EE (30 % after 180 days) than when Gly was used as co-solvent (Figure 5b). This is because even though the emulsions are thermodynamically unstable, and the presence of crystalline material or impurities could promote nucleation and instability during storage, substances including co-solvents as
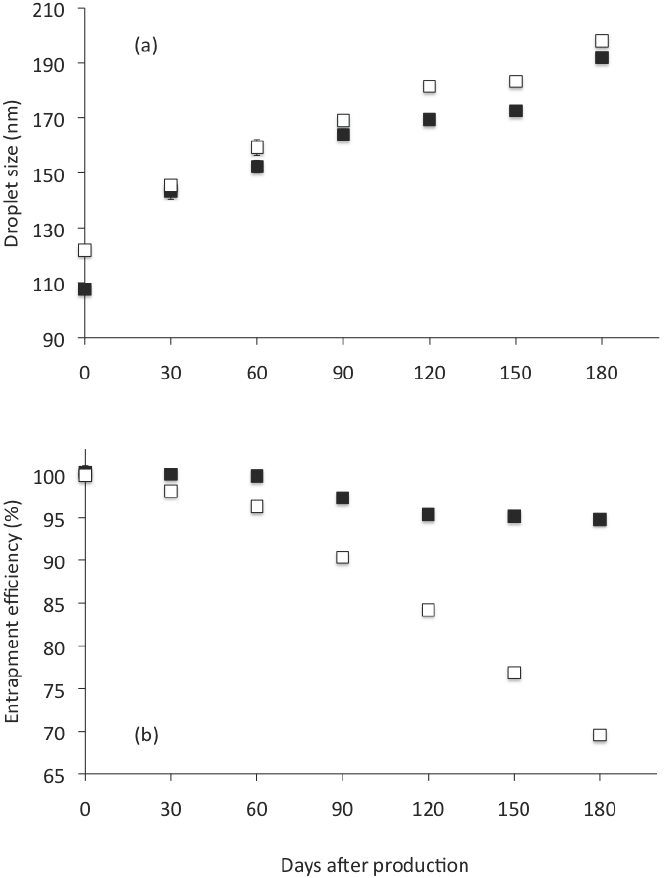
Figure 5 Droplet size (a) and entrapment effciency (b) of CN analyzed right after preparation and intervals until 180 days storage at 4 °C. Open square: formulation without glycerol. Closed square: formulation with glycerol. Corresponding to formulations FTFHES 3 and FTFHES 4 in Table 2 . Preparation method: Thin-film hydrationemulsification and ultrasonication. Data represent the mean ± standard deviation of 2 independent experiments (n = 2).
Gly, are able to inhibit nucleation and may be useful for preventing undesirable nucleation, crystal growth, and precipitation of a bioactive component (Warren et al., 2010). Gly increased the micro- viscosity of the continuous phase, slowed down the diffusion of solute molecules through the solution, thereby delaying their incorporation into the solid- liquid interface, and thus promoted physical stability.
Conclusions
This study showed that as composition (emulsifier concentration, co-solvent addition, and bioactive component loading) as preparation method (EES or TFHES) employed to form CN had effects on entrapment efficiency, mean droplet size, polydispersity index, and stability. The entrapment efficiency of C into CN was favored by Gly addition in the aqueous phase of NE and its preparation by TFHES. The CN prepared by this method, using 5 % w/w of MCO, 10 % w/w of SL, and 42.5 % of Gly showed an entrapment efficiency of 100 % and were stable for 120 days at 4°C. When increasing amounts of C beyond 2.5 mg per g were used, the bioactive incorporated in the emulsion became saturated.











 nueva página del texto (beta)
nueva página del texto (beta)