1 Introduction
Mexico has the largest diversity of genetic resources of maize in the world. Many of them are pigmented genotypes as purple, red, black and yellow and they have been identified as appropriate for the masa and tortilla industry (Sanchez et al., 2000; Vázquez-Carrillo et al., 2011). Pigmented maize kernels and nixtamalized corn flour prepared from them contain many secondary metabolites, such as phenolic compounds, anthocyanins among others. Strong evidence of high content of anthocyanins, phenolic compounds and antioxidant capacity has been reported for pigmented maize genotypes (Adom & Liu, 2002; Mora-Rochin et al., 2010; López Martinez et al., 2009). These compounds have attracted the consumer attention due to their potential health benefits (Liu, 2004). Phenolic acids and flavonoids represent the most common form of phenolic compounds found in whole maize kernel, with a number of types that exist as soluble free and conjugated or insoluble bound forms (Zilic' et al., 2012). Furthermore, since anthocyanins are flavonoids, they are water- soluble glycosides of polyhydroxyl and polymethoxy derivates of 2-phenylbenzopyrylium or flavylium salts. They are found in the pericarp and aleurones. Anthocyanins determine the color of pigment maize and these water-soluble compounds are potent natural antioxidants due to their ability to trap free radicals (Stavric, 1994).
Acrylamide, a neurotoxic compound (Spencer & Schaumburg, 1974) and possible human carcinogen (IARC, 1994), has been found in high concentrations in thermally processed foods (Tareke et al., 2002). Its formation is related to Maillard reaction and specifically with the presence of carbonyl compounds with groups capable of forming a Schiff Base with the asparagine amino acid (Hidalgo et al., 2009)
Numerous studies have also been directed to find antioxidants that minimize acrylamide content. However, the role of antioxidants on acrylamide formation in foods is still controversial and both positive (Kalita et al., 2013; Cheng et al., 2015) and negative or null effects (Ac ̧ar & Go ̈kmen, 2009; Cai et al., 2014) have been described. This is likely a consequence of both, the wide diversity of antioxidant compounds and mixtures, and the different systems in which they can be assayed. Therefore, the potential effect of each antioxidant, or extract, should be assayed in the system in which it might be potentially useful (Salazar et al., 2012).
Antioxidants are able to prevent or reduce reactions of lipid oxidation; therefore, the compounds responsible for the color in pigmented maize can be potentially used to reduce acrylamide formation in foods thermally processed from nixtamalized pigmented corn flour, because they can inhibit the formation of carbonyl compounds responsible to convert asparagine into acrylamide.
Based on the health risks involved in the consumption of acrylamide, the development of alternatives aimed at reducing the levels of this compound in foods broadly consumed by the population are required. Thus, in an attempt to investigate the use of pigmented maize as an option to produce tortilla chips with reduced levels of acrylamide, this work studies the formation of acrylamide in fried tortilla made from nixtamalized white, black, red, purple and yellow maize.
2 Materials and methods
2.1 Materials
Labeled [2,2,3-2 H3 ]acrylamide was purchase from Sigma-Aldrich (St. Louis, MO). All other chemicals were analytical grade and purchased from Sigma (St. Louis, MO) or Merck (Darmstadt, Germany). Soybean oil was obtained from local supermarkets in Querétaro, México.
Five types of maize were chosen: a white maize hybrid (Pioneer 30P16) and four pigmented maize landraces with yellow, red, purple and black colors grown in Querétaro, México.
2.2 Methods
2.2.1 Elaboration of nixtamalized corn flour and tortilla chips
Nixtamalized corn flour (NCF) was prepared with the types of maize above-mentioned and commercial lime (Ca(OH)2) (El Topo, Monterrey, N.L. Mexico), commonly used in the tortilla industry. The flour was prepared by cooking 8 kg of whole corn kernels in a solution of 16 liters of water with 80 g of Ca(OH)2, corresponding to 1.0 g/100 g of lime relative to the corn weight used. The corn was boiled in an aluminum pan for 23-25 min and steeped for 16 h at room temperature (22 ± 1°C). The steep liquor was removed. The cooked corn was washed with 16 liters of water, then ground into corn dough (FUMASA, M100, Querétaro, México), and finally dehydrated using a flash type dryer (Cinvestav-AV, M2000, Querétaro, México). The drying conditions were adjusted to have 250°C inlet air temperature and 90°C to the exhaust air to avoid burning the material. Before storage, the nixtamalized corn flour was milled using a hammer mill PULVEX 200 (Molinos Pulvex, S.A. de C.V., México DF) equipped with a 0.5 mm screen.
For tortilla chips elaboration, nixtamalized corn flour (100 g) was rehydrated with enough water (118 mL) to provide fresh dough with proper consistency to make tortillas. The dough was shaped into thin disks (11 cm diameter and 1.0 mm thickness) using a commercial tortilla roll machine. The dough shaped into tortillas was cooked on both sides for around 1.0 min by using an iron hot plate (270 ± 10 °C). The resulting tortillas were cut into circular pieces with an average area of 10 cm2. Tortilla pieces were fried in soybean oil at 180°C for 30 and 45 s. The 30 s time was used because in preliminary tests, this time was found as the best time to produce tortilla chips. A frying time of 45 s was also assayed to analyze the effect of extended frying times on acrylamide formation. After frying, tortilla chips were cooled on a paper towel to remove superficial oil and the color, breaking force, and acrylamide content determined.
2.2.2 Proximate analysis
The experimental NCF were analyzed in triplicate, and the protein (P), fat (F) and ash content (A) as well as pH values were determined using standard methods of the American Association of Cereal Chemists (AACC, 1997). Crude fiber content (CF) was evaluated by the 962.09.43 method (AOAC, 1997).
2.2.3 Determination of total anthocyanins content
Total anthocyanins (TANT) were assayed according to the method of Abdel-Aal and Hucl, (1999) by measuring the absorbance of methanolic extracts. One gram of nixtamalized corn flour was homogenized with 20 mL of an acidified methanol solution (95%) and 1 M HCl 85:15 v/v in a centrifuge tube. The tube was flushed with nitrogen gas, agitated for 30 min and then centrifuged at 2000 x g for 15 min and the supernatants were collected. For the quantification, a 2 mL aliquot of the extract was measured spectrophotometrically at 535 nm, the wavelength at which anthocyanins exhibit maximum absorption. Anthocyanins were expressed as mg of cyanidin-3 glucoside equivalents/kg sample dry basis. For quantification was used a molar extinction coefficient of 25965 M−1 cm−1 and a molecular weight of 449.2 g/mol (Abdel-Aal & Hucl, 1999).
2.2.4 Determination of total phenolic content
The free (FPH) and bound phenolic (BPH) content in nixtamalized corn flours were extracted according to the procedure described by De la Parra et al., (2007) and modified in our laboratory. Briefly, one gram of nixtamalized corn flour was blended with 10 mL of 80% chilled ethanol for 10 min and then centrifuged at 2000 x g for 15 min. The supernatant was removed and stored at -20 °C until used.
BPH compounds of NCF were extracted from the residue of the extraction above-mentioned according to the method reported by De la Parra et al., (2007). The residue was digested with 10 mL of 2 M sodium hydroxide at room temperature; the tube was flushed with nitrogen gas, and the sample was shaken for 1 h. 10 mL of water were added and the mixture pH was adjusted to 2 with hydrochloric acid and extracted with hexane to remove lipids. The final solution was extracted with 20 mL of ethyl acetate. The ethyl acetate fraction was evaporated to dryness. BPH compounds were reconstituted in 10 mL of distilled water. The extracts were stored at 4 °C until used.
FPH and BPH content on NCF extract samples were quantificated using the Folin-Ciocalteau method described by Singleton et al., (1999). The standard was gallic acid and the results were expressed in mg of gallic acid equivalents /100 g of sample dry basis.
2.2.5 Color determination in tortilla chip samples
Color changes were determined using a colorimeter MiniScan XE, model 45/0-L (Hunter Associates Laboratory, 11491 Sunset Hill Rd., Reston, Va., U.S.A.). Total color differences (∆E) at different periods of time were calculated from the determined CIELAB L* a* b* values according to Hunter (1973): ∆E = [(∆L*2 + (∆a*)2 + (∆b*)2]1/2; where L* = brightness or lightness (100 = perfect white, to 0 = black); a* = greenness/redness [negative (green) to positive (red)]; b * = yellowness/ blueness [negative (blue) to positive (yellow)]; ∆L*, ∆a*, and ∆b* = absolute differences of the values between the reference tile (white porcelain) and sample values; ∆E = total difference between reference and sample color. The reference values (calibration) were: L* = 92.22, a* = -0.82 and b* = 0.62.
2.2.6 Texture determination in tortilla chips
The fracture force of the tortilla chips was evaluated using the Texture Analyzer TA-XT2 (Texture Technologies Corp., N. Y.). Fracture force was evaluated in freshly prepared samples. The test was carried out using a 2.03 mm diameter stainless- steel probe and a platform accessory with a hollow cylindrical base with 33.5 and 10 mm external and internal diameters, respectively. The probe traveled at a velocity of 10 mm/s to a depth of 6 mm until it cracked the sample. The results were recorded in N.
2.2.7 Acrylamide determination in tortilla chips
Acrylamide was analyzed as the stable 2- bromopropenamide derivative by gas chromatography- mass spectrometry (GC-MS) as described previously (Salazar et al., 2012) with some modifications. Briefly, tortilla chips were ground in a mortar lab and powdered samples (~ 0.8 g) were successively weighed in centrifugal tubes, spiked with 20 μL of internal standard solution (0.5 mg/mL of deuterium- labeled [2,2,3-2 H3 ]acrylamide in acetonitrile), and stirred with 8 mL of distilled water and 10 mL of n-hexane at room temperature for 5 min. After centrifugation at 2000 x g for 10 min, organic phases were removed. Co-extractives from supernatants were precipitated with 30 μL of Carrez I and 30 μL of Carrez II solutions. Later, supernatants were centrifuged at 2000 x g for 5 min and filtered. These extracts (4 mL) were treated with 0.45 g of potassium bromide, 200 μL of sulfuric acid (10 mL/100 mL), and 300 μL of potassium bromate solution (0.1 mol/L). After 1 h in the dark at 4°C, the bromination reaction was terminated by adding of 1 mol/L sodium thiosulfate until solutions became colorless, and solutions were extracted with 5 mL of ethyl acetate/hexane (4:1). Organic layers were recovered after centrifugation at 2000 x g for 10 min, and were dried with sodium sulfate and evaporated to dryness under nitrogen. Each sample was dissolved in 50 μL of ethyl acetate, treated with 25 μL of triethylamine, and analyzed by GC- MS. The ions monitored for the identification of the analyte, 2- bromopropenamide, were
GC-MS analyses were conducted with a Perkin Elmer GC Clarus 500 coupled with a Perkin Elmer Clarus 560 MSD (Mass Selective Detector- Quadrupole type). In most experiments, a 30 m × 0.32 mm i.d. × 0.25 μm Elite-5MS capillary column was used. Working conditions were as follows: carrier gas helium (1 mL/min at constant flow); injector, 250°C; oven temperature: from 60 (10 min) to 130°C at 5°C/min and then to 300°C at 10°C/min; transfer line to MSD, 280°C; ionization EI, 70 eV.
Quantification of acrylamide was carried out by preparing standard curves of this compound. Acrylamide content was directly proportional to the acrylamide/internal standard area ratio (r = 0.999, p< 0.0001). The coefficients of variation at the different concentrations were lower than 10%.
2.2.7 Statistical analysis
All results were expressed as mean ± SD values (n=3). When significant F values were obtained, group differences were evaluated by the Tukey's test. Pearson's correlation was used to identify the association between the dependent variables. All statistical procedures were carried out using the JMP 9.0 package (SAS Institute Inc., Cary, NC). The significance level was p< 0.05 unless otherwise indicated.
3 Results and discussion
Texture and color are considered the most important parameters of quality and acceptability of fried products. Figure 1 shows the effect of frying time on the parameters of tortilla chips above-mentioned. The color has been correlated with the acrylamide generation in thermally processed foods. Regardless of the frying time, the appearance of the tortilla chips (Figure 1A) does not show significant changes between each type of pigmented maize used. Overall, the fracture force (Figure 1B) showed similar values over the range of frying time studied. The results showed that tortilla chips made of pigmented maize kernels had a similar texture from those made of commercial maize (white).
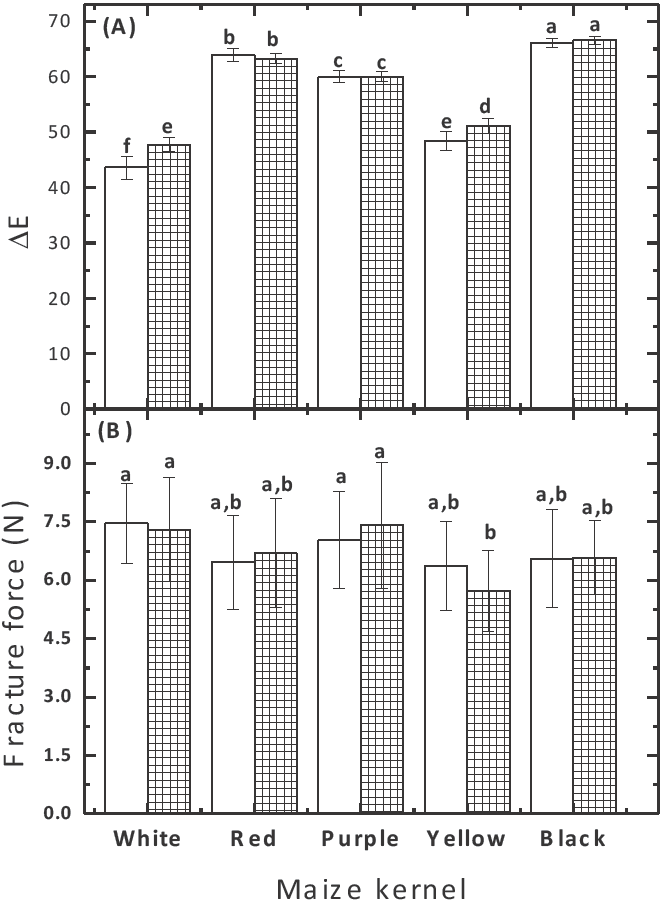
Fig. 1 Effect of maize kernel used to prepare nixtamalized corn flour on: color (A), and fracture force (B) in tortilla chips fried at 180°C for 30 (nonhatched) and 45 (crosshatched bars) seconds. Bars with different letters are significantly different (p< 0.05).
Table 1 shows significant variations in the nutritional composition of the NCF prepared from different pigmented maize kernels. Flours from yellow, black, red and white maize showed the greatest A content (17.82 -18.85 g/kg). Moreover, with the exception of the white maize (22.60 g/kg), all maize assayed produced nixtamalized flours with similar values of CF (28.13 to 29.88 g/kg). Higher pH values were registered in yellow and white NCF. In addition, the greatest F content (57.92 g/kg) was showed by yellow genotype being this parameter relevant for acrylamide formation in fat-rich foods as it was suggested by Capuano et al., (2010) and Hidalgo et al., (2010) due to the role of lipid oxidation in acrylamide formation.
Table 1 Chemical composition of flours processed from pigmented nixtamalized corn.
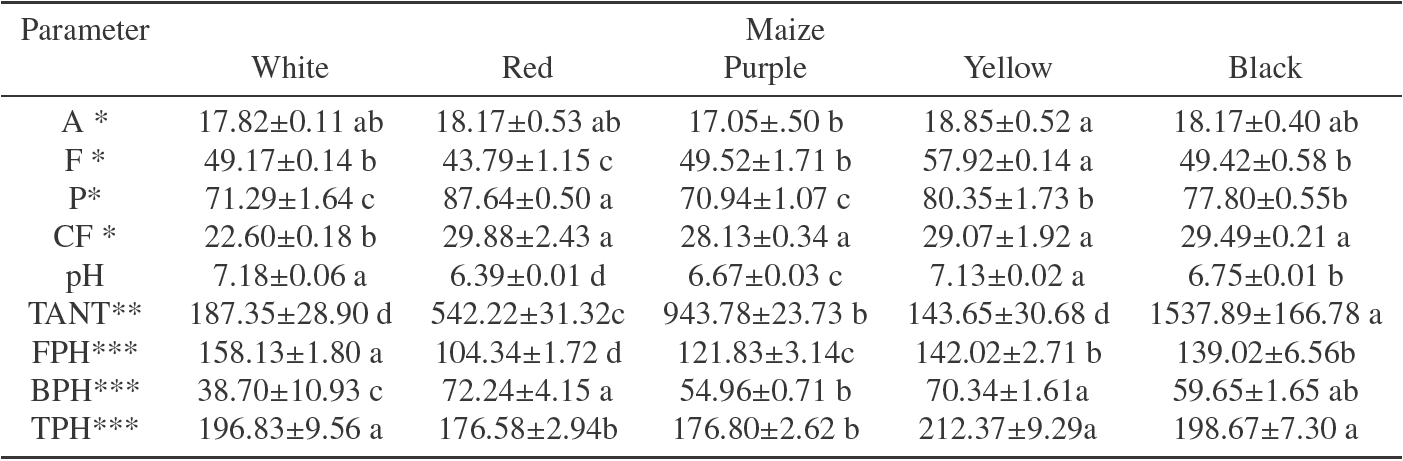
A= ash; F= fat; P= protein; CF= crude fiber; pH= pH value; TANT= total anthocyanins; FPH= free phenolic; BPH; bound phenolic; TPH= total phenolic
Mean ± standard deviation. Means values followed of different letter, in the same row, are significantly different (p<0.05)
* Concentration expressed in g/kg dry basis
** Concentration expressed in mg cyanidin-3 glucoside equivalents/kg dry basis
*** Concentration expressed in mg gallic acid equivalents/100 g dry basis
On the other hand, flours from red maize showed the greatest P content (87.64 g/kg) which could be related to the content of asparagine, one of the major precursors of acrylamide formation (Mottram et al., 2002). Independently of the maize used, chemical composition of the NCF obtained in this study was in accordance to those reported previously for commercial and ecological ones (Campechano- Carrera et al., 2012).
The maize genotype used significantly affected both the phenolic compounds and anthocyanin content. The differences observed can be attributed to seasonal environmental, geographical growing conditions, the physical properties of the grain and the relative relation of the pericarp and endosperm, which are the richest structures in those compounds (Salinas-Moreno et al., 2003). Moreover, although most of the phenolics are bound or attached to cell wall structures, many of them were lost during lime- cooking by leaching into the step solution or nejayote.
The range of total phenolic content (TPH) for flour was from to 176 to 212 mg gallic acid equivalents/100 g. The range of FPH content was from 104 to 158 mg gallic acid equivalents/100 g. The range of BPH content was from 38 to 72 mg gallic acid equivalents/100 g. Although BPH content is lower than values reported previously (De la Parra et al., (2007); Mendez et al., 2013), as mentioned above, the differences can be attributed to the alkaline and thermal treatment conditions used in this study during the nixtamalization process and the lixiviation of the phenolic compounds in the cooking solution which greatly influenced on the loss of them. Thus, the highest phenolics content was found in the yellow NCF while the lowest was determined in red and purple NCF.
TANT content ranged from 143 to 1537 mg cyanidin-3 glucoside/kg for the five studied NCF. As it was expected, flours made of yellow and white showed the lowest anthocyanin content indicating that carotenoids, as it has been suggested, particularly in the form of lutein and zeaxanthin, are the most abundant pigment type in these maize genotypes (Kurilich & Juvik, 1999). Among the group of pigmented flours, the black one had the greatest anthocyanin levels. However, flours abundant in phenolics were not the most abundant in anthocyanins. According to Lopez-Martinez et al., (2009) in maize isolates with a greater proportion of anthocyanins, a higher levels of antioxidant activity is expected.
Figure 2 shows acrylamide content in tortilla chips, prepared from NCF with different pigmented maize kernels. All flours induced the formation of acrylamide in the tortilla chips being the acrylamide content similar to levels founded in potato products (Ou et al., 2008; Lineback et al., 2012). Samples prepared from yellow NCF always contained more acrylamide (p < 0.05) (>600 μg/kg) than those prepared from pigmented maize kernels flours. The results could be a consequence of the amino acid composition of maize and reducing sugars. Previous studies have showed a correlation of reducing sugars and asparagine with the acrylamide content on fried products of potato tubers with colored flesh (Kalita et al., 2013) and commercial potato varieties (Zhu et al., 2010).
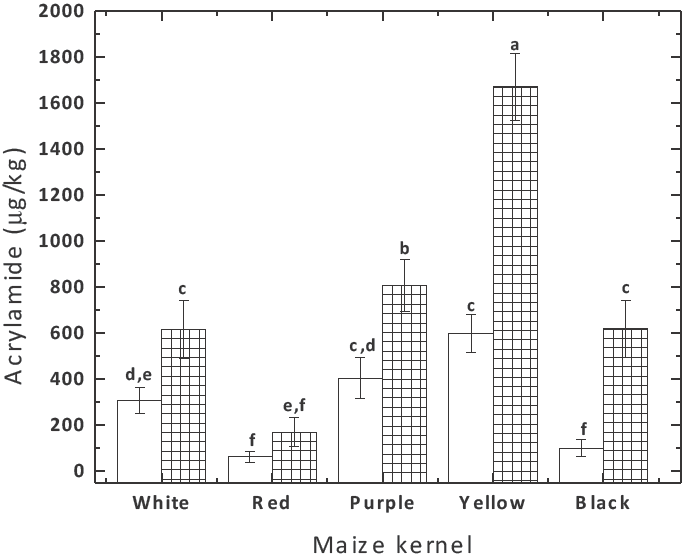
Fig. 2 Effect of maize kernel used to prepare nixtamalized corn flour on acrylamide content in tortilla chips fried at 180°C for 30 (non-hatched) and 45 (crosshatched bars) seconds. Bars with different letters are significantly different (p< 0.05).
These results above-mentioned were independent of the frying time. The only significant difference found was the amount of acrylamide produced by some pigmented maize genotypes (which increased with the frying time, as expected). For tortilla chips fried for 30 s the average amount of acrylamide formed in red (62.92 μg/kg) and black (101.79 μg/kg) was the lowest as compared to white, purple and yellow nixtamalized corn flours.
When tortilla chips were fried for 45 s, a non-significant increase on acrylamide content was observed in tortilla chips made of red NCF. In contrast, the acrylamide level in tortilla chips prepared from the remaining flours was approximately doubled.
Some authors suggested that antioxidant compounds including medicinal plant extracts, phenolic acids, and flavonoids could influence the Maillard reaction, which then affects acrylamide formation (Cheng et al., 2009; Zhu et al., 2009) Taking in to consideration the phenolic acids and anthocyanins as factors that influence acrylamide formation; it was studied acrylamide formation in tortilla chips prepared from antioxidant-rich pigmented maize. In this study, TPH as well as FPH and pH value were positively correlated with acrylamide formation (Table 2). On the other hand, a negative relationship was observed among the content of TANT with the acrylamide levels in tortilla chips. The selection of maize genotypes rich in anthocyanins as well as lower levels of fat and phenolics could reduce acrylamide formation in tortilla chips and other tortilla-based foods thermally processed.
Table 2 Correlation coefficients* between chemical composition of flours and acrylamide content in tortilla chips.
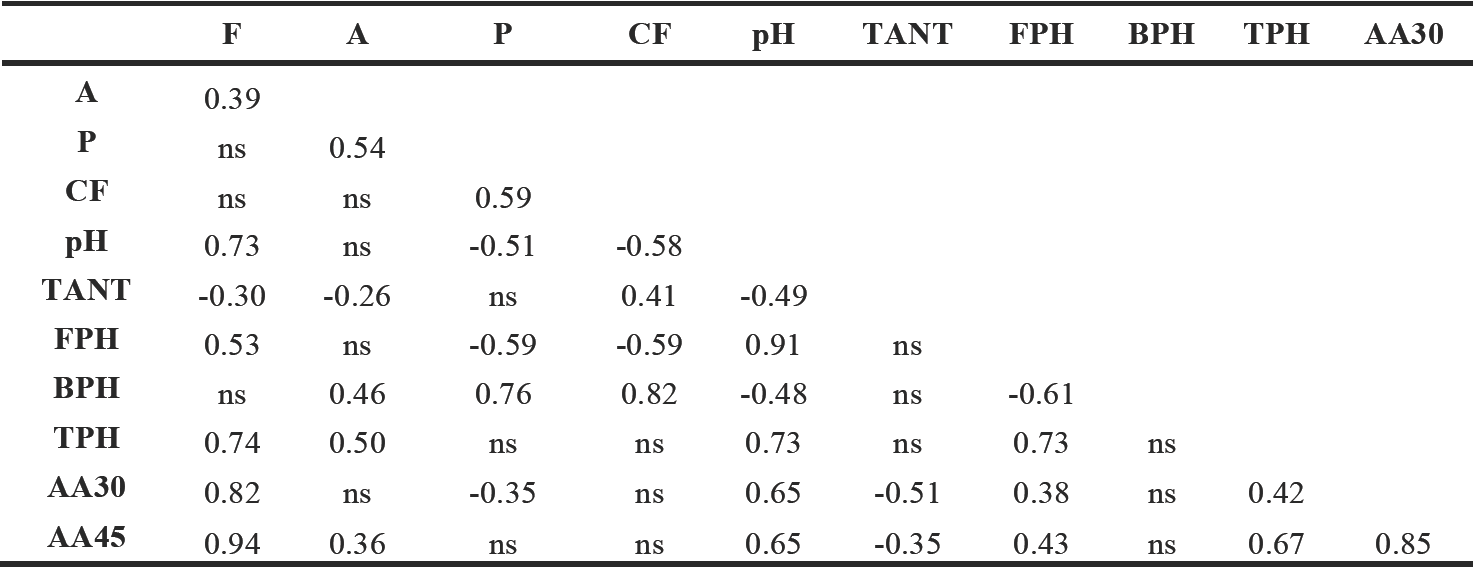
A= ash; F= fat; P= protein; CF= crude fiber; pH= pH value; TANT= total anthocyanins; FPH= free phenolic; BPH; bound phenolic; TPH= total phenolic; AA30= acrylamide content in tortilla chips fried 30 s; AA45= acrylamide content in tortilla chips fried 45 s
* Significant at p < 0.05; ns= no-significant.
The role of fats in acrylamide formation has been probed by several authors (Capuano et al., 2010; Hidalgo et al., 2010). They pointed out that some oxidized lipids are able to convert asparagine into acrylamide. The strong correlation coefficient of acrylamide and fats found in this study (0.82 and 0.94, in tortilla chips fried for 30 and 45 seconds, respectively) is in agreement with this fact. Further studies in the fat composition of the different flours might elucidate this relationship opening new possibilities of control of acrylamide formation in tortilla chips manufactory.
On the other hand, the influence of antioxidant on acrylamide formation was found to be contradictory. Several studies have reported the effect of phenolics on acrylamide formation but both positive and negative results had been obtained (Jin et al., 2013). The same antioxidant compound showed difference mitigating acrylamide effect in a model system or food matrix depending on the study. For instance, Bassama et al., (2010) found that the addition of pure phenolic compounds such as caffeic acid, catechin, cinnamic acid, ferulic acid, coumaric acid, gallic acid, and epicatechin to the aqueous model system did not mitigate acrylamide formation, whereas Ou et al., (2010) studied the effect of several antioxidant compounds, including tert-butylhydroquinone (TBHQ), butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), ferulic acid, and vitamin C, and found a reduction or elimination of acrylamide when antioxidants were added. Kotsiou et al., (2011) focused on the chemical structure more than antioxidant capacity of the phenolic compound assayed, suggested that the terminal functional groups (hydroxyl or aldehyde) of the side chain might play a significant role in affecting the phenols' capability to interrupt or enhance certain steps of the pathway to the formation of acrylamide. Recently, Liu et al., (2015) stated that the controversial effects of the polyphenols on acrylamide formation were related to their structure, concentrations, and antioxidant capacity, as well as reaction conditions. While some polyphenols can inhibit acrylamide formation by trapping of carbonyl compounds, and so preventing against lipid oxidation, other polyphenols can enhance the acrylamide content by providing carbonyl groups, accelerating the conversion from 3-aminopropionamide to acrylamide and inhibiting acrylamide elimination. In this study, tortilla chips prepared from red and black maize have relatively less acrylamide than purple, yellow and white maize, which may be due to the presence of higher bound phenolics and anthocyanins. Nevertheless, additional studies are needed to investigate the role of the red and black maize composition (asparagine and reducing sugars content) on the formation of acrylamide in tortilla chips prepared from pigmented maize kernels.
Conclusions
The level of acrylamide generated in tortilla chips was significant positive correlated with fat content and phenolic compounds in pigmented nixtamalized corn flour. Furthermore, this study reported a relationship between the acrylamide formation and the total anthocyanins content in flour, and revealed that a higher level of anthocyanins in pigmented maize kernels might reduce acrylamide formation in tortilla during frying. Although further studies about the content of phenolic compounds after frying are needed, preliminary results showed that the selection of suitable red and black pigmented maize can reduce the level of acrylamide formation improving nutritional characteristics of foods in which pigmented kernels may be used in their formulation. This is of the upmost importance, because it provides the food manufacturer with information of which maize pigmented genotype is more likely to get a low acrylamide content product over similar processing conditions used in this study.











 text new page (beta)
text new page (beta)


