1 Introduction
Tannase or tannin acyl hydrolase (3.1.1.20) catalyzes the hydrolysis reaction of ester bonds present in gallotannins, complex tannins, and gallic acid esters (Mukherjee, 2007). This enzyme is used for quality improvement of beer and wine production. Tannase is used for treatment of juice and solid residues of grape. In addition, this enzyme is employed to clarify some fruit juices and for tannin elimination of contaminants in effluents of the leather industry (Aguilar et al., 2001; Ramírez et al., 2008). Other important use of this enzyme is for production of propyl gallate in the food industry and trimethoprim in the pharmaceutical industry (Lekha and Lonsane, 1994). Actually, tannase is used as an indicator of colon cancer, manufacture of cosmetics, treatment of agroindustrial wastes for ethanol production, and others (Aguilar-Zarate et al., 2014). Tannase has been identified in animals, vegetables and microorganisms. This last one is the most important source because the produced enzyme is more stable; it is produced in great amounts, in constant form, and placed under fermentation techniques, enzyme production and titers of enzymatic activity increase (Lekha and Lonsane, 1997).
Since Hatamoto et al. (1996) described the tannase sequence from A. oryzae, which had 1767 bp and 588 amino acids with a signal peptide of 18 amino acids, new source of tannase has been searched. Cruz-Hernández et al. (2005) isolated different tannin-degrading strains from Mexican semi-desert plants and soils. Two of the most important isolated strains are Aspergillus niger GH1 and PSH, which have been reported as tannase producer strains in both submerged (SmF) and solid state fermentation (SSF) (Belmares et al., 2003; Mata-Gómez et al., 2009; Flores-Maltos et al., 2011). According to the above, the purpose of the present study were identifying, expressing and sequencing the tannase gene from two Aspergillus niger GH1 and PSH strains and stablishing genetic relationship of the obtained sequences with other reported sequences of tannase.
2 Materials and methods
2.1 Strains, medium composition and chemicals
Aspergillus niger GH1 and PSH strains were obtained from the DIA-UAdeC culture collection. The fungal strains were propagated using the Czapek medium which was prepared with the following composition (g/L): KH2 PO4 (2.19), (NH4)2SO4 (4.38), MgSO4·7H2O (0.44), CaCl2·7H2O (0.044), MnCl2·6H2O (0.009), NaMoO4·2H2O (0.004) and FeSO4·7H2O (0.06). These reagents were obtained from Productos Químicos Monterrey (Monterrey, Nuevo Leon, Mex). Tannic acid, methytl gallate and gallic acid were obtained from Sigma-Aldrich Co. (St. Louis, MO).
2.2 Submerged fermentation
Aspergillus niger GH1 and PSH strains were grown on potato dextrose agar (PDA) at 30° C for 6 days. Fungal spores were harvested using 0.1% Tween 80 and were counted in a Neubauer chamber. 1 × 106 spores / mL were used as inoculums. Erlenmeyer flasks (250 mL) with 30 mL of Czapek medium and tannic acid (25 g/L) were employed as reactors. The pH was adjusted at 5.0, inoculum of 1 × 106 spores / mL was added to the culture medium. Reactors were incubated at 35°C and shaked in a multi-wrist Shaker system at speed 4 and 250 rpm. Kinetic of tannase production was monitored for 72 h and samples were taken every 12 h. The experiment was carried out in duplicate.
2.2.1 Tannase assay
The filtered enzymatic extracts were dialyzed with a buffer of citrate (pH 5.0) for 24 h using a cellulose membrane. The tannase activity was determined employing the technique reported by Sharma (2000). Briefly, in this step, it was used 0.01 M methytl gallate as substrate, which was prepared in 0.05 M citrate buffer (pH= 5.0). In addition, acid gallic (100 ppm) was used as standard. Absorbance was measured at 520 nm using a Thermo Spectronic Biomate spectrophotometer. One unit of tannase was defined as the amount of the enzyme able to release one μmol of gallic acid per minute under the assayed conditions (reaction time: 25 min. and temperature: 30°C).
2.2.2 Protein assay
Protein content was measured using Bradford (1976) technique. Serum bovine album at 1000 ppm was used as standard. Absorbance was measured at 595 nm using a Thermo Spectronic Biomate spectrophotometer.
2.3 PCR protocol for tannase gene amplification
Fungal biomass was produced in Czapek medium, using glucose (30 g/L) as carbon source. Inoculum of 200 μL was added to Erlenmeyer flask of 250 mL, which contained the culture medium. The flask was incubated at 200 rpm, at 30°C during 5 days. Subsequently, the biomass was washed three times with distilled sterile water, then biomass was frozen using liquid N2. Fungal biomass was macerated to obtain a white and fine powder. DNA extraction technique used TES extraction buffer (50 mM pH 7.5 Tris-HCl, 20 mM EDTA and 1% SDS) as reported by Barth and Gallardin (1996). Some modifications were realized; the addition of PEG was eliminated from the technique. On the other hand, DNA washes were performed with phenol/chloroform/isoamyl alcohol (25:24:1), addition of 7.5 M ammonium acetate and cold 100% ethanol (v/v) was included. Polymerase Chain Reaction (PCR) was performed as follows: initial denaturation at 95°C for 5 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 57°C for 1 min and extensión at 72°C for 1 min. Then, a final elongation at 72°C for 5 minutes was done. TAN1 (5'-gRTAgAACTggTACCA- 3') and TAN 2 (5'-ACCTCTCCTggCAgATYg-3') oligonucleotides at 10 mM, previously designed (Cerda-Gómez, 2006) were employed for PCR protocol. Paq5000 DNA Polymerase (5U/μL) Agilent Technologies was used in this procedure. DNA of Aspergillus niger Aa-20 was used as positive control. This strain was reported previously as tannase producer.
2.3.1. Sequencing and bioinformatics analysis
Sequencing reactions were performed by Sanger method and using TAN1 and TAN2 oligonucleotides. The obtained sequences were analyzed in Bioedit (Hall, 1999) and Blastn 2.2.27 (Zhang et al., 2000; Larkin et al., 2007). Finally, phylogenetic analysis was conducted using Mega 5.1 (Tamura et al., 2011).
2.4 Tannase gene expression
Other submerged culture was performed using the conditions mentioned previously. Samples were monitored every 6 h. Biomass was collected and RNA was extracted using Trizol Reagent. Then, cDNA was obtained by RT-PCR according to Durón-Vázquez (2011). Similarly, PCR was done as mentioned above.
3 Results and discussion
Tannase production by A. niger GH1 was detected and its maximum activity was 344 U/L at 24 h of culture. In the next hours, a decrease was observed (Figure 1). This behavior is due to proteolysis as described by Aguilar et al. (2002) whom observed that to high tannic acid concentration (100 g/L), this activity is minor; and at low tannic acid concentration, the protease activity of A. niger Aa-20 was high in the culture medium. Other authors have reported the tannase activity from different Aspergillus niger isolates, where the enzymatic activity is higher than that obtained in the present study (Table 1). This tendency may be due to inoculums size, tannic acid concentration and fermentation time. However, similarly results were obtained by De la Cerda et al. (2011) whom used A. niger GH1 for tannase production. Considering these factors, tannase production should be optimized. By the way, specific activity was calculated, in this case 4 U/mg of protein were showed at 24 h of SmC. Minor values of protein has been reported for A. niger AUMC 4301 of 0.038 U/ mg protein at 5 days of incubation (El-Fouly et al., 2010).
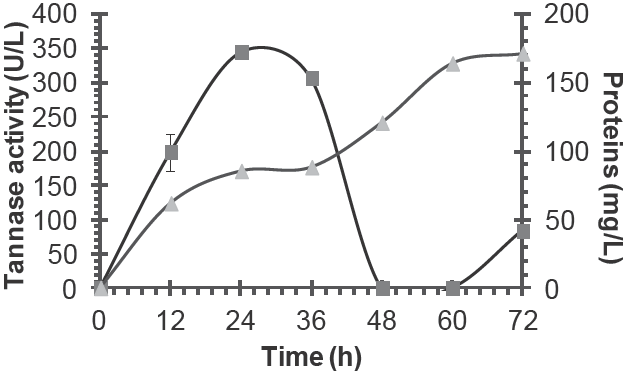
Fig. 1 Tannase (■) and proteins (▲) secretions detected during Submerged Culture from Aspergillus niger GH1.
A single band of 412 pb was detected after the PCR and RT-PCR analyses (Fig. 2a and 2b, respectively), which corresponded to a fragment of tannase gene. Alignments realized in Blastn 2.2.28+ of the tannase sequences obtained in the present study demonstrated that PSH (310 pb) and GH1 (298 pb) sequences had 97% similarity with A. niger strain SL-5(Acc. No. JN848716.1) begins at 893position. In addition, similarity of 95% with A. niger CBS 513.88 (Acc. No. XM 001402449.1) in 1106 position was detected; this is confirmed by genome sequencing of the strain, according to Pel et al. (2007). Other values found: 91% with Aspergillus awamori strain BTMFW032 (Acc. No.GQ337057.2) (Beena et al., 2010) and 87% with tannase from A. niger (TanAni) (DQ185610.2) begins in 2928 nucleotide. Meanwhile, the gene expression was detected after 18 h (Figure 2b) in SmC, assuming a decrease at 36 h, this can be confirmed with the tannase activity decrease at the same time (Figure 1) by reasons discussed before. The phylogenetic relationship for homogeneity assumption was constructed (Figure 3). Sequence of A. niger GH1 and PSH were grouped with A. niger strain SL- 5, this is interpreted as higher similarity and common ancestor for the gene.
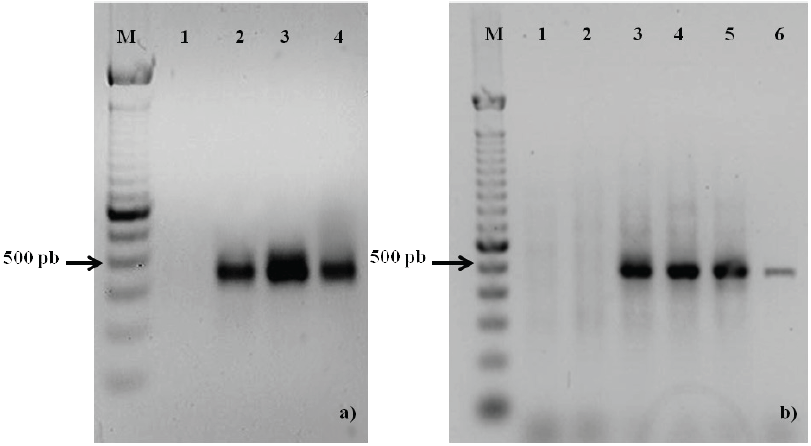
Fig. 2 PCR amplification of a tannase gene fragment (a). M: molecular size marker, 1: water nuclease free as negative control, 2: positive control (Aa-20), 3 and 4 GH1 and PSH DNA genomic, respectively. (b) Tannase expression at different times during SmC. 1) 6 h; 2) 12 h; 3) 18 h; 4) 24 h; 5) 30 h and 6) 36 h. Electrophoresis was done using 1 % (w/v) agarose gel, TAE-1X buffer and the gel was stained using ethidium bromure.
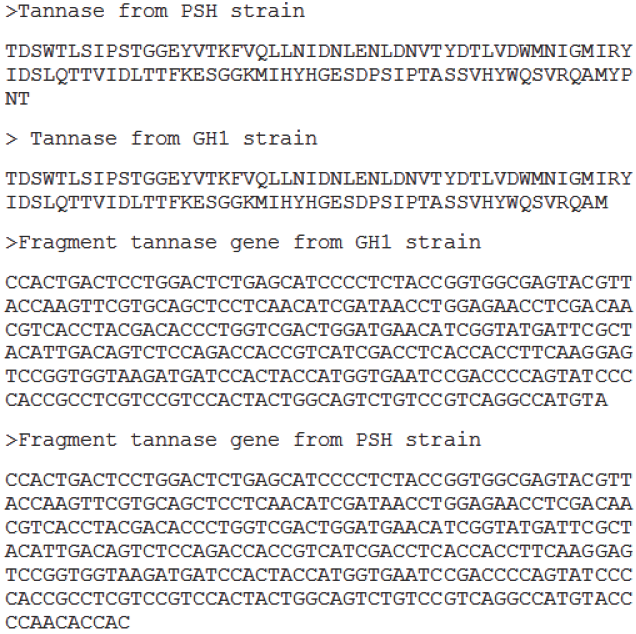
Fig. 3 Phylogenetic relationship among tannase sequences from Aspergillus species reported in GenBank and the tanasse sequences from A. niger GH1 and PSH obtained in the present study. NJ model with 1000 bootstrap re-sampling and Tamura 3- parameter substitution model was employment using MEGA 5.1 program.
Finally, amino acid sequence were aligned, the 4, 42 and 91positions are detected as Trp, which is localized in core proteins and play an important role on its stability and it is a very conserved codon, thus, it is difficult that present a mutation. However, if a mutation is showed, generally, Trp is substituted by other aromatic amino acid such as Phe and Tyr, as in 4 and 92 positions. Tannase sequences were evaluated in Motif Scan (Sigrist et al., 2010). First, sequence 12-99 amino acids were confirmed that our sequence corresponded to tannase and feruloyl sterase, the last enzyme is involved in cosmetic and health industries (Camacho-Ruiz et al., 2014). Position between 63- 65 and 93-95 are denominated as protein kinase C phosphorylation site. In addition, positions between 11-14, 38-41, 57-60 and 63-66 are recognized as cistein kinase II phosphorylation sites. Protein kinases are responsible for proteic function regulation; the post translation modification is fast and reversible (Campbell et al., 2007). These data were confirmed using NetPhos 2.0 tool (Blom et al., 1999), where seven phosphorilation sites were detected in GH1 tannase sequence: 2 of Ser (10, and 67 positions), 3 of Thr (38, 56 and 63 positions) and 2 of Tyr (50 and 90 positions). Those amino acids are very reactive because of its hydroxyl group.
Conclusions
Tannase activity by A. niger GH1 was observed using Submerged Culture with the maximum activity at 24 h. In addition, a fragment of tannase gene from A. niger GH1 and PSH was identified by PCR and RT-PCR. These genes are highly similar to those reported previously. Tannase amino acid sequence was confirmed by bioinformatic tools and was identified as tannase and feruloyl sterase with sites of proteic function regulation. Future studies are necessaries for isolate the full tannase gene and to study its biochemical properties.











 nueva página del texto (beta)
nueva página del texto (beta)



