1 Introduction
The transformation of the raw material in agricultural production processes, forestry and agribusiness present low efficiency which gives rise to a number of misused and discarded wastes. The vegetable biomass is considered a potential industrial valuable material known as lignocellulose; it is mainly composed by cellulose, hemicellulose and lignin molecules (González-Rentería et al. 2011; Howard et al. 2003).
Around 1550 million tons of agricultural waste is annually generated in the world, while in Colombia around 72 million tons of agricultural biomass residues is annually generated (Dashtban et al. 2009; Escalante et al. 2011; MADR, 2006). Ramírez-Carmona (2012) conducted the agricultural biomass residues map in Antioquia's province, finding a maximum value of residual biomass of 93.72 ton/ha cultivated for 92 species.
Fique (Furcraea macrophylla) is a native plant from South-American Andean region, located within the countries of Colombia, Venezuela and Ecuador (Casierra-Posada and Gómez, 2008; Cauca Government, 2002). In Colombia, fique is cultivated for the extraction of a natural fiber called "cabuya", in which extraction process generates juice and bagasse wastes with contents of active industrial potential value ingredients (Ramírez, 2008).
In Antioquia province around 1495 hectares of Fique are grown, whose performance represents 2356 tons per year of cabuya, generating 600 t/year of lignocellulosic agricultural residue called fique spall (MADR, 2010; Cía. de Empaques S. A., Personal Comunication, March 18th 2012).
The use of lignocellulosic wastes is subject to the preparation and purification of any of its components. Cellulose obtaining lignocellulosic wastes depends on the degradation of the lignin present in the polymer matrix that covers and protects the cellulose microfibers (Casey, 1990; Colonna, 2010; Sánchez, 2009; Van Dyk and Pletschke, 2012).
The separation of cellulose from other lignocellulosic components is performed by physical and chemical process that seeks to degrade lignin and hemicelluloses that accompany it (Bozzalla, 2010).
A biological alternative to the lignin degradation content in the fique spall is the use of ligninolytic microorganisms. The principal bodies described the ability of degrade and mineralize lignin is a group of Basidiomycetes causing the "White Rot" disease on wood (Papinutti et al. 2003).
These organisms synthesize enzymes on Laccase, Manganese-Peroxidase and Lignin-Peroxidase to degrade lignin molecule to simple chemical compounds that introduce in their metabolism as a carbon source (Fengel and Wegener, 2003). These enzymes have low specificity catalytic mechanism, so can mineralize a wide range of organic molecules (Lebrun et al. 2011). Extracellular enzymes cleave Ether and carbon-carbon linkages that hold type basic lignin monomer by oxidative processes (El Mansouri, 2006; Sánchez, 2009).
The use of fermented broths or crude enzyme extracts from lignin-degrading microorganisms is a simple and e↵ective method for the cellulose separation, compared to the direct use of microorganisms, such as the absence of celluloses enzymes that degrade cellulose to separate, also the diculties of microorganism growth on a large scale or long incubation processes (Karam and Nicel, 1997).
In this investigation, the cellulose separation of ligniocellulose contained in the fique spall using different ligninolytic "White Rot" fungi fermented broth was performed by enzymatic degradation. The degradation eciency of lignin by three ligninolytic enzymes of Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineous was evaluated.
2. Materials and methods
2.1 Microorganisms and growth medium
Fungi Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineous were used for the fermentation. The microorganisms were provided by Centre for Studies and Research in Biotechnology (CIBIOT) of the Universidad Pontificia Bolivariana of Medellín, Colombia.
In growth medium (MCL) for preservation of microorganisms and fermentations were prepared using lignin of cane bagasse as a Carbon source (10 g/L); NH4NO3 (2 g/L); K2HPO4 (0.8 g/L); MgSO4 (0.5 g/L); Yeast Extract (2 g/L) and NaPH2O4 (0.75 g/L).
2.2 Growth kinetics
The growth kinetics of fungi Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineous were determined by measuring the CO2 production (mg/L) in a system OXITOP Box 115 WTW. Kinetics were performed in 100 mL of growth medium MCL, inoculum of 1 mL spore suspension for 160 h.
2.3 Delignification in the culture medium
Delignification in the growing medium was performed by the lignin kinetics of fermented broth production. Initially, 1 mL of each suspension was inoculated fungus: Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineous in 100 mL of growth medium MCL in 250 mL Erlenmeyer flasks. Samples were taken every 24 h for a period of 96 h. The samples were filtered under vacuum using a membrane filter (Schleicher and Schuell, 100) to separate the biomass from the fermented broth. The fermented broth was centrifuged at 4000 rpm (BoecoGermany, U-320) to take the supernatant and filtered again with a filter of 0.45 μm pore size. Subsequently, lignin was quantified.
2.4 Delignification of fique spall
For the enzymatic degradation of lignin contained in the fique spall, 100 mL of fermented broth filtered was added to 1 g of fique spall in 250 mL Erlenmeyer flasks at pH 5.0, temperature of 50 °C and magnetic stirring at a constant speed of 150 rpm for 30 min. Thereafter, vacuum filtration using a membrane filter (Schleicher and Schuell, 100) to separate the fique spall treated from the fermented broth was performed. The solid residue was dried in an oven (1DIES, VCA115) to consistent weight. Thereafter lignin and Laccase, Manganese-Peroxidase and Lignin- Peroxidase enzyme activities were measured.
Having demonstrated the capacity to degrade lignin in the fique spall, cellulose content was determined after enzymatic treatment with fermented broth of Pleurotus sp.
2.5 Cellulose content
The Cellulose content in the fique spall was determined by reading High Resolution Liquid Chromatograph HPLC (Shimadzu). The chromatographic separation was performed with a Waters IC-Pack ion exclusion column and refractive index detector Shimadzu, RID-10A model. It was used a mobile phase H2SO4 (0.005N) at a pumping flow rate of 0.6 mL/min.
2.6 Lignin content
The lignin content in the fique spall was determined by the Klasson technique (H2SO4 al 72%) for the acid insoluble fraction. For determination of the fermented broth, it was filtered using a membrane filter (Schleicher and Schuell, 100). The filtrate was dried in an oven (1DIES, VCA115) at 105 °C to constant weight. The material obtained was subjected to extraction of soluble solvent extractor (Velp Scientifica, SER-148), using ethanol-toluene and distilled water as solvents.
After extracted soluble, acid digestion of the sample was performed following the method Klasson lignin, employing H2SO4 (72 %) relative 1:0.1 (H2SO4: sample) for a period of 90 min to 30°C. It was then diluted reducing the concentration of sulfuric acid up to 3 % (w/w) by adding distilled water. This solution was placed in an autoclave at 121 °C for 1 h and then filtered using a membrane filter (Schleicher and Schuell, 100). The solid residue was dried in an oven (1DIES, VCA115) to constant weight to obtain the acid insoluble lignin content value.
2.7 Enzyme activity assays
The enzymatic activity of the fungal Pleurotus sp. CBT1, Pleurotus sp. CBT2 y Pycnoporus sanguineous in growth medium MCL for Laccase, Manganese-Peroxidase and Lignin-Peroxidase was determined.
Quantification of enzyme activity was performed by determining the absorbance in a UV/VIS Spectrophotometer (Shimadzu, UV-1601PC) for three enzymes. Quartz and glass cuvettes of 3 mL as reaction volume were used.
The enzymatic activity of the Laccase enzyme was determined as described Bourbonnais et al. (1995). 50 μL sample, 1850 μL de Buffer de Sodium Acetate 100 mM (pH 5.0) and 400 μL reactive Acid 2,2'-azino-bis (3-etilbenzotiazolin-6-sulfonico) (ABTS) 1 mM were taken. The reaction lasted for 1 min. ABTS oxidation was monitored by the increase in absorbance at 420 nm, using absorptivity (s) 36000 M/cm.
The enzymatic activity of the Manganese- Peroxidase enzyme was determined as described Field et al. (1992). 1800 μL sample, 600 μL Buffer Sodium Malonate 250 mM (pH 4.5) and 150 μL 2,6-Dimetoxiphenol (DMP) 20 mM were taken. The reaction lasted for 2 min. 150 μL MnSO4.H2O was added. The reaction lasted for 2 min. Then, the Mn2+ influence on the Mn-P activity was measured for 2 min. Thereafter 300 μL of H2O2 was added and absorbance was measured for 3 min. DMP oxidation was followed by the increase in absorbance at 468 nm using absorptivity (s) 18300 M/cm.
The enzymatic activity of the Lignin-Peroxidase enzyme was determined as described Kirk and Tien (1988). 900 μL sample, 1200 μL de Buffer Sodium Tartrate 250 mM (pH 3.0) and 600 μL Veratryl Alcohol (10 mM). The reaction lasted for 1 min. Thereafter 300 μL of H2O2 was added and absorbance was measured for 1 min. Veratryl Alcohol oxidation was followed by the increase in absorbance at 310 nm using absorptivity (s) 9300 M/cm.
Enzyme activity was expressed as International Units per volume unit (IU/L).
3. Results and discussion
3.1 Growth kinetics
Pleurotus sp. CBT1, Pleurotus sp. CBT2 and P. sanguineus showed growth kinetics in MCL medium according to the characteristic pattern of microbial growth kinetics (Madigan et al. 2003) and showed a similar ability to grow in this type of environment, reaching population levels corresponding to a production of maximum CO2 close to 200 mg/L (Figure 1).

Figure 1 Growth kinetics in terms of CO2 (mg/L), of Pleurotus sp. CBT1, Pleurotus sp.CBT1, Pleurotus sp. CBT2 and P. sanguineus in MCL culture medium, for a period of 160 h.
The lag phase for Pleurotus sp. CBT1 lasted approximately 15 h, while for P. sanguineus and Pleurotus sp. CBT2 was 20 and 30 h, respectively. This may mean a greater ability of the organism to synthesize the enzymes needed to catabolize the lignin molecule present in the culture medium and incorporate the carbon to its metabolism, initiating the population increase.
Similarly, Pleurotus sp. CBT1 evidenced a rapid increase in its population over the next 40 h. Then, the growth gradually decreases until the end of the assay. It can be inferred that between 24 and 72 h of fermentation, the microorganism is able to get the necessary nutrients for their survival and reproduction. This ability is restricted in the following 72 h due to the limitation of an essential nutrient or the accumulation of any inhibitory waste product.
The behavior of the growth for the microorganisms tested is within the range of values reported by other authors for different fungi known for their ability to degrade lignin (White Rot Fungi) and similar molecules (Chaparro and Rosas, 2006; Osorio and Quintero, 2009; Díaz, 2009; Henao et al. 2006).
Studies by Chaparro and Rosas (2006) reported a lag phase around 24 h for Earliella sp, Cookeina sp, Xilaria sp, three ligninolytic species isolated from wood. Cardona et al. (2009) obtained lag phases with 10 and 15 h of duration for Phanerochate sordida and Phanerochate chrysosporum, respectively, in a culture medium composed by wheat bran and glucose-malt extract, respectively. Similarly, Pleurotus ostreatus took a period of at least 100 h for adaptation to malt extract medium (Diaz, 2009).
3.2 Delignification of the culture medium
Delignification of the culture medium was determined by lignin content during the fermented broth production kinetics. The initial content of lignin in the culture medium was 33%. Lignin degradation began between 12 and 24 h after the start of fermentation for the three species. This coincides with the end of the lag phase of microorganisms (20 h) on the growth kinetics, reinforcing the hypothesis about the ligninolytic enzymes synthesis by microorganisms during this growth phase (Fig. 2).
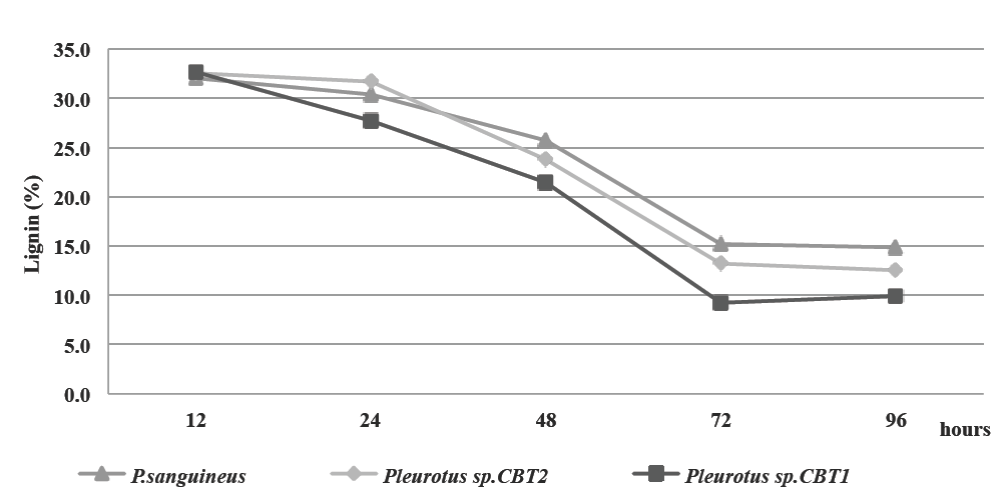
Figure 2 Lignin content (%) in MCL culture medium for all three microorganisms. all three microorganisms.
72 h after fermentation, all three species obtained the maximum lignin degradation. Lignin content decreased to 15.18% for P. sanguineus, 13.2% for Pleurotus sp. CBT2 and 9.24% for Pleurotus sp. CBT1. The highest lignin degradation was obtained during the exponential growth phase for the three microorganisms. In this phase the ligninolytic enzymes necessary to depolymerize the principal source of carbon in the culture medium was produced in larger quantities.
For the period of the subsequent 72 h, the lignin degradation is reduced, although the microorganisms population growth is still increasing.
Generally, Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineus showed a lignin degradation eciency that exceeded 50 %. Pleurotus sp. CBT1 was the species with the higher lignin depolymerization level, evidencing an eciency of 72%. This coincides with a best response of this species in their growth kinetics compared to the other species tested (Fig. 3).
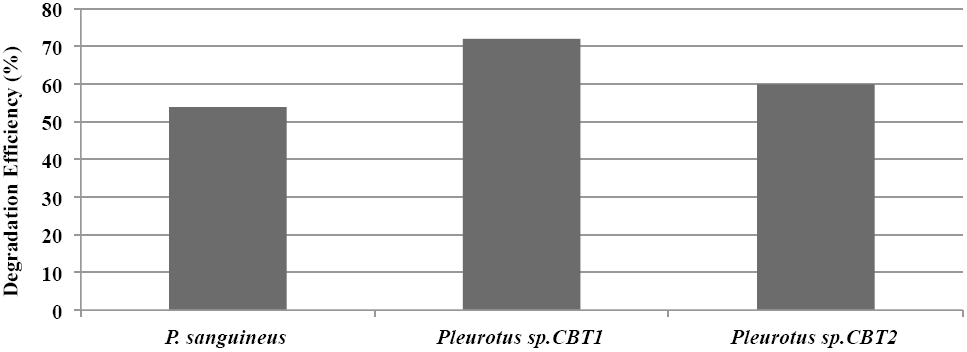
Figure 3 Máximum Lignin degradation efficiency (%) in culture medium for Pleurotus sp. CTB1, Pleurotus sp. CTB2 and P. sanguineus.
Studies with Pleurotus sp. have shown greater ability to degrade aromatic compounds like lignin, compared to microorganisms known for their ligninolytic ability. Sahoo and Gupta (2005) obtained aromatic dyes degradation levels higher to 80%, 72 hours after fermentation, while Trametes versicolor and Phanerochaete chrysosporium, known ligninolytic fungi, only reached around 60% values. Meanwhile, MacDonald (2012) obtained a modification of lignin of 30% using Phanerochaete chrysosporium, while that Antai and Crawford (1981) obtained a lignin loss of 31.9% using different strains of Streptomyces sp. in herbaceous plants.
Lignin degradation by Pleurotus sp. CBT1, Pleurotus sp. CBT2 and Pycnoporus sanguineus reflects their ability to synthesize extracellular ligninolytic enzymes, which interact with the lignin polymer through oxidation reactions resulting in the molecule (Bertrand, et al. 2013; Dashtban et al. 2009; Feng et al. 2011; et al. 2012; Lebrun et al. 2011; Howard et al. 2003; Papinutti et al. 2005; Pinto et al. 2012).
The depolymerization of lignin molecule by ligninolytic fungi or bacteria begins outside of the microorganism. There, peroxidase enzymes (Lignin- Peroxidase and Manganese-Peroxidase) and phenol oxidase enzymes (Laccase) break ether bonds (α-O-4, β-O-4, 4-O-5) and Carbon-Carbon bonds, generating lower molecular weight compounds, which can be dilignoles and/or monolignols (Dashtban et al. 2009; Feng et al. 2011; Fackler et al. 2006; Gassara et al. 2012; Lebrun et al. 2011; Howard et al. 2003; Papinutti et al. 2005; Pinto et al. 2012).
Once finished the extracellular enzymatic depolymerisation, the residual lignin oligomers are mineralized into the microorganism through a metabolic pathway that leads to cellular respiration processes (Sylvia et al. 2005)
3.3 Delignification of the fique spall
The initial content of lignin in the fique spall was 14.1% (control). The fermented broth of Pleurotus sp. CBT1 and Pleurotus sp. CBT2 and P. sanguineus showed ability to degrade lignin contained in the treated material. This behavior was obtained under conditions of pH 5.0, temperature 50 °C temperature, and fique spall concentration of 1% and 30 min of enzymatic action time.
In Figure 4, the behavior of the lignin content in the fique spall (in terms of percentage of lignin) after the enzymatic treatment with the fermented broth of the three microorganisms for a period of 96 h is shown.
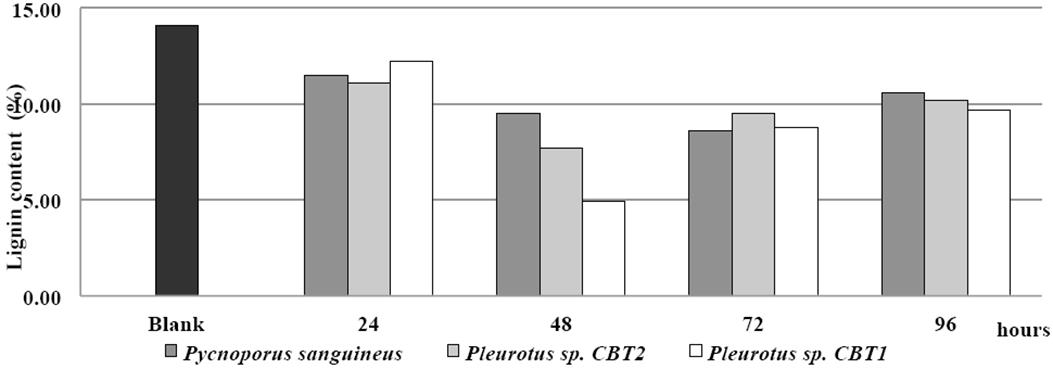
Figure 4 Lignin content (%) in fique spall before and after enzymatic treatment with fermented broth for all three species.
24 h after fermentation, the fermented broths of all three organisms presented a ligninolytic activity on the fique spall. Lignin degradation for this period was about 12% for the three species.
48 h after fermentation, the fermented broth begins to show di↵erences in the degradative capacity of the three species. For this period, the lignin content in the fique spall is less than 10%, reaching levels of 4.9 and 7.7% in the case of Pleurotus sp. CBT1 and Pleurotus sp. CBT2, respectively. These values are the greater levels of lignin reduction in the fique spall for these two species. P. sanguineus reached a lower content of lignin in the fique spall (8.6%) with a 72 h fermented broth.
Pleurotus sp. CBT1 fermented broth provided further lignin degradation in the fique spall than those of Pleurotus sp. CBT2 and P. sanguineus. Lignin content of fique spall fell to 4.9% with the fermented broth of Pleurotus sp. CBT1. This value represent a reduction of the lignin content on fique spall of 20 and 26 % higher than Pleurotus sp. CBT2 and P. sanguineus, respectively.
Varnaite and Raudoniene (2005) conducted tests using the full microorganism, reporting a decrease in lignin content in rye straw (16.2% initial) to 8.4 and 9.4% using Galactomyces geotrichum and Myrothecium verrucaria respectively.
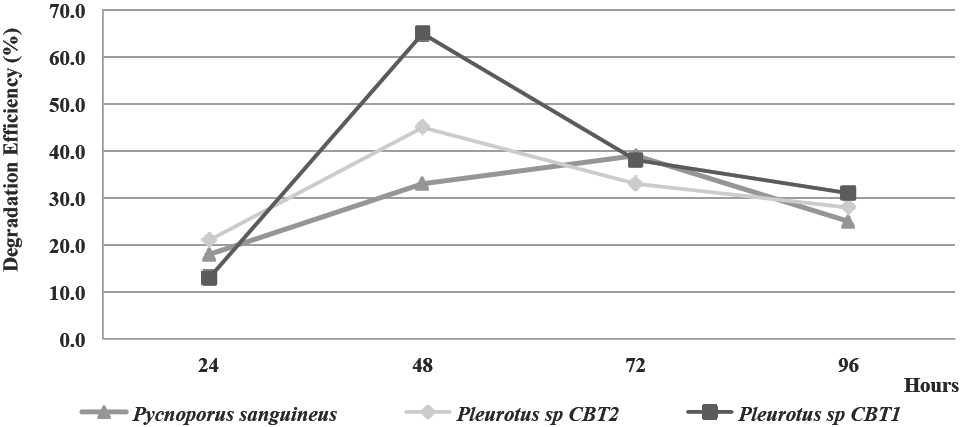
In Figure 5 the behavior of lignin degradation eciency on fique spall by all three organisms, in terms of percent degradation is shown.
Maximum levels of lignin degradation on fique spall for the fermented broths of Pleurotus sp. CBT1 and Pleurotus sp. CBT2 was reached at 48 h of fermentation. These levels were of 65 and 45% of lignin degradation, respectively. The highest eciency for P. sanguineus was presented with the fermented broth of 72 h (39%).
This results are similar to those obtained in studies in which used fermented broth of ligninolytic fungi. Husaini et al. (2011) achieved lignin degradation efficiency up to 25% in 60 days by solid state fermentation using Acacia magnium wood with crude extracts of Coriolus versicolor and Pycnoporus cockiness. Meanwhile, Yu et al. (2006) reached 80% of decolorization by degradation of aromatic components from textile Red Dye K-2BP, using the fermented broth of 72 h of Phanerochaete chrysosporum.
Figure 6 shows the enzymatic activity in terms of International Units (IU/L) of Laccase, Manganese-Peroxidase and Lignin-Peroxidase in the fermented broths of Pleurotus sp. CBT1, Pleurotus sp. CBT2 and P. sanguineus and their lignin degradation efficiency on fique spall.

Fig. 6 Enzymatic Activity (IU/L) of Laccase, Manganese-Peroxidase and Lignin-Peroxidase in MCL culture medium and lignin degradation efficiency in fique spall (%) for Pleurotus sp. CBT1 (A), Pleurotus sp. CBT2 (B) and P. sanguineus (C).
The lignin degradation eciency on fique spall is directly related to the activity of the ligninolytic enzymes present in the fermented broth for all three organisms, particularly with Mn-peroxidase. The decrease of enzymatic activity of Mn-P coincides with a lower response in the lignin degradation on the fique spall for fermented broths after 48 h for Pleurotus sp. CBT1 and Pleurotus sp. CBT2 and after 72 h for P. sanguineus.
In 2009, Pant et al. reported similar results for Aspergillus sp. in the degradation of aromatic components of textile dyes using its fermented broth. These authors obtained a decolourisation percentage close 30% when was presented with a high enzymatic activity of Mn-P (4.5 IU/L).
The maximum enzymatic activity of Mn-P in the fermented broth of Pleurotus sp. CBT1 and Pleurotus sp. CBT2 was submitted after 48h of fermentation, coinciding with the maximum lignin degradation efficiency. In contrast, the higher efficiency of degradation for P. sanguineus fermented broth appears to 72 h, period at which the enzymatic activity of Mn-P starts decreasing.
The enzymatic activity of Laccase and Lignin-Peroxidase was lower than 0.5 and 2.0 IU/L, respectively for the three microorganisms throughout the test, keeping in line with evidenced in previous assays.
Having demonstrated the ability to degrade lignin, the cellulose contact of fique spall was determined after enzymatic treatment with fermented broth of tus sp. CBT2 (B) and P. sanguineus (C). Pleurotus sp. CBT1, as the micro-organism showed higher lignin degradation (65%).
After enzymatic treatment with the fermented broth, the cellulose content in the fique spall increased to 73.1% (Figure 7).

Fig. 7 Lignin and Cellulose content in fique spall before and after enzymatic treatment with fermented broth of Pleurotus sp. CBT1.
The increase in the proportion of the cellulose confirms the selective lignin degradation in fique spall by action of ligninolytic enzymes present in the fermented broth. It can be inferred that the lignin presence and the cellulose absence in the culture medium during the production of fermented broth redirected the metabolism of the microorganisms to the synthesis of ligninolytic enzymes in detriment of celluloses, so that the cellulose content is not affected during treatment.
Conclusions
The fermented broth of Pleurotus sp. CBT1 after 48 h of fermentation had higher lignin degradation in the fique spall than those ones of Pleurotus sp. CBT2 and P. sanguineus, decreasing the initial lignin content of 14.1% to 4.9%. This representing an increase in the cellulose content in the spall of 61.8 % to 73.1 % after treatment.
The lignin biodegradation allows increasing the cellulosic fraction in agricultural wastes. Therefore, this process is useful to the cellulose exploitation as a raw material or the natural cellulose based materials development.











 nueva página del texto (beta)
nueva página del texto (beta)