1 Introduction
Solid state fermentation (SSF) holds tremendous potential for the production of enzymes. Agro- industrial by-products such as lignocellulosic biomass can be employed as low-cost support and/or substrates in SSF. However, several plant polysaccharides are partially esterified with acetic and hydroxycinnamic acids (which is one of its natural roles to protect plant cell walls against invading microorganisms) which can hinder hydrolysis. Indeed, hydrocinnamic acids such as ferulates are esterified to the C5 α-hydroxyl of -L-arabinose side chains and hemicelluloses chains (xylan) could be cross-linked by oxidative coupling of ferulate monomers. These diferulate cross-linking of xylans are thought to structurally impede the enzymatic degradation (Grabber et al. 2000).
Carbohydrate esterases (CEs) are enzymes catalyzing O- or N-deacylation of substituted saccharides (i.e. esters or amides) and they are also able to hydrolyze esters in which sugars plays the role of an acid (Biely, 2012). Xylanolytic esterases hydrolyze ester linkages between xylose units and acetic acid in the xylan chain (acetyl xylan esterase EC 3.1.1.72) or between arabinose side chain residues and hydroxycinnamic acids (feruloyl esterase EC 3.1.1.73) (Christov & Prior, 1993). Acetyl xylan esterases (AcXEs) have been classified into the carbohydrate esterase family CE1, together with some feruloyl esterases (FAEs), and into families CE2- CE7 and CE16 (Cantarel et al., 2009). Many FAEs do not fit into the established CE families and they have been separately classified into four subclasses (types A-D), based on their ability to hydrolyze four different methyl hydroxycinnamates (i.e. ferulate, caffeate, sinapinate and p-coumarate methyl esters). Their ability to release diferulic acids from esterified substrates and their sequence homology, which is the most common way to classify this kind of enzymes (Crepin et al., 2004). However, recently (Udatha et al., 2011) proposed a new classification scheme which display FAEs into 12 different families, based on FAE- related amino acid sequences from bacteria, fungi and plants.
FAEs and AcXEs have an important role in animal nutrition (Cao et al., 2013), the pulp and paper industry (Record et al., 2003), and in the breakdown of plant biomass for the production of bioactive phytochemicals and biofuels (Faulds et al., 1997; Tabka et al., 2006; Zhang et al., 2011). FAEs have gained increased attention in the area of biocatalytic transformations for the synthesis of bioactive compounds for cosmetic and health applications (Moussou et al., 2004; Vafiadi et al., 2008). FAEs and AcXEs have been recognized as common components of hemicellulolytic enzyme systems of many microorganisms (Williamson et al., 1998). The two major FAEs of A. niger have received special attention, namely AnFaeA and AnFaeB (Faulds, 2009); these enzymes have been classified as type A and type C, respectively (Crepin, Faulds et al., 2004); and into the sub-families 12A and 4A of the FAE families, respectively (Udatha, Kouskoumvekaki et al., 2011). The expression of these enzymes may be regulated depending on the substrate used for fermentation (de Vries et al., 2002), even using different agro-industrial by-products (Crepin, Faulds et al., 2004). Both fae genes are known to be induced in the presence of different subset of aromatic compounds (de Vries et al., 2002). The selective expression of these genes is desirable, due to the specificity of the synthesized enzymes, because a particular enzyme is required to perform a specific reaction.
The use of agro-industrial by-products as carbon and energy sources offers the advantage of combining the use of a cheap substrate and an interesting way of adding value to a by-product. In Mexico, millions of tons of residues from grain crops are generated per year (Valdez-Vazquez et al., 2010). In 2010, about 23 million tons of corn (one of the most cultivated grains), 3.6 million tons of wheat and 254 thousand tons of coffee were produced in Mexico (INEGI, 2012). Residues generated by these grain crops are hardly, if ever, used. Additionally, some of the residues represent an increasing environmental problem.
A. niger FAEs and AcXEs have been mostly produced using wheat bran (Johnson et al., 1988; Donaghy & McKay, 1995; Koseki et al., 1998), sugar cane bagasse (Johnson, Harrison et al., 1988; Garcia et al., 1998) and sugar beet pulp (Donaghy & McKay, 1995; Kroon et al., 1996). However FAE production has been poorly studied using corn bran and coffee pulp as carbon sources. It is still unclear what type of FAE is produced using these kinds of substrates as carbon source. Moreover, AcXEs production had not been studied in these by-products. The aim of this work was to evaluate the effect of different local agro-industrial by-products on high and preferential production of FAEs and AcXEs. For this purpose A. niger PCS6 was cultivated on corn bran, coffee pulp and wheat bran as substrates by SSF.
2 Materials and methods
2.1 Reagents
All solvents and chemical compounds used in this study were purchased from Sigma-Aldrich, Toluca, México, except Czapek-Dox-agar, which was acquired from Merck, Darmstadtt, Germany; malt-extract agar and potato dextrose agar (PDA) were obtained from Difco, Detroit, USA.
2.2 Raw material
Used corn bran (CB), the outer layer of the kernel, was kindly donated by Corn Products and Ingredients, S.A. de C.V., Guadalajara, Jalisco, Mexico; coffee pulp was provided by Diversificados Argovia S.A. de C.V, Tapachula municipality, Chiapas, Mexico; and wheat bran (WB) was obtained from Forrajes Tesistán, S.A de C.V. These agro-industrial by-products were grinded and sifted to a particle size between 0.180 and 0.425 mm.
2.3 Phenolic acid extraction of agro- industrial by-products and HPLC analysis
0.5 g of dry coffee pulp, wheat and corn bran were incubated during 4 h in 5 mL methanol:ethyl acetate 1:1 (v:v) at 40 °C. The organic phase was totally removed by centrifugation and used for the free phenolic acids analysis. The remaining solid pellets were hydrolyzed overnight using 5 mL of 1N NaOH at 25 °C. After hydrolysis and centrifugation the solid pellets were again incubated during 15 min in 10 mL of 4N NaOH at 121°C and pH values in the alkaline aqueous phases were adjusted to pH 2 using 4N HCl. Phenolics were extracted 3 times from both samples, using 20 mL of ethyl acetate and used for the ester and ether phenolic acids analysis, respectively (Mattila & Kumpulainen, 2002). The phenolic acids identification and its quantification was performed by High Performance Liquid Chromatography (HPLC), using a Varian ProStar 230 chromatograph with UV detection at 300 nm, on a ZORBAX SB-C18 column (250 x 4.6 mm, 5 micron, Agilent technologies, Palo Alto, CA). The flow rate was constant during each run (0.4 mL min−1). An eluent composition of 20:80 (v:v) acetonitrile:water was held for 9 min, followed by a gradient increase 75:25 (v:v) acetonitrile:water for 3 min, where the eluent composition was maintained for the final 8 min of the run. Finally, an equilibrating time of 5 min was applied.
2.4 Strain isolation, classical identification, and conservation
Aspergillus PCS6 was isolated from superficial coffee pulp monticules as previously described (Ramirez et al., 2008). The classical identification was performed by cultural and morphological approaches. First, the strain was grown on Czapek-Dox agar and malt- extract agar to confirm purity and species were identified based on macro and micro-morphological criteria (Klich, M., 2002). For further fermentation experiments, the strain was grown and maintained on Potato Dextrose agar (3.9%, w v−1) at 4 °C.
2.5 Molecular identification of fungal strain
The strains PCS6 was grown on PDA at 30 °C for 72 h. Subsequently, fungal mycelium was collected by scraping the surface of the solid culture and DNA was extracted with the commercial kit Dneasy® Plant Mini Kit (Quiagen, Hilden, Germany) following the manufacturer's instructions. PCR was carried out with OneTaq® DNA polymerase (New England Biolabs) on a Veriti T M 96-well thermal cycler (Applied Biosystems, Foster City, USA). Primers ITS1 (5' TCC GTA GGT GAA CCT GCG G 3') and ITS4 (5' TCC TCC GCT TAT TGATAT GC 3') were employed to amplify an approx. 600 bp fragment from the ribosomal gene cluster under the same reaction conditions described previously (Segura-Garcia et al., 2010). PCR products were sent to Macrogen USA Corp. for sequencing of both strands. Consensus sequences were obtained with the CLC Main Workbench 5.5 software package (CLCBio, Aarhus, Denmark) and compared to recorded sequences from GenBank database using the BLAST algorithm (Altshul et al., 1990).
2.6 Solid state fermentation conditions
SSF was performed as previously described (Rodriguez et al., 2006). Corn bran, coffee pulp or wheat bran were used as solid substrates. The support (cubed polyurethane 0.5 cm edge) was mixed with the solid substrate at a 25:75 (w:w) ratio, and impregnated with a minimal salt solution (pH 6.5): urea 1.2 g, K2HPO4 1.5 g and MgSO4 0.3 g per 100 g of dry matter. Glucose (1.5 g per 100 g of dry matter 100 g) was added as starter. The solid impregnate was sterilized at 121 °C for 15 min, and inoculated with 2 × 107 conidiospores of A. niger PCS6 g−1 of dry matter adjusting the moisture content of the media to 75% (w w−1) with deionized water. Conidiospores of A. niger PCS6 was produced according to Reyes- Ocampo et al. (2013). Glass columns (2.5 × 30 cm) were packed with inoculated solid media, and were incubated at 30 °C, in a controlled water bath. A constant stream of water-saturated air was supplied to the bottom of the columns at 40 mL min−1. Generally, two columns were sampled daily during fermentation experiments. These columns were stored at -20 °C for further analysis. The fermented solid matter of each column was crumbled and homogenized by mixing thoroughly. The enzyme was extracted from 14 g of fermented solid by mechanically pressing using a manual squeezer. This extract was centrifuged (5000 × g, 5 min, 4 °C) and the supernatant was used as the crude enzyme sample, in which esterase activity was determined.
2.7 Synthesis of the methyl hydroxycinnamates
Hydroxycinnamic acids (4.85 g of ferulic acid, p- coumaric acid, caffeic acid or sinapinic acid) and 50 mL of methyl alcohol containing 4% of hydrogen chloride, were placed in an egg-plant type flask (200 mL), and heated under reflux for 1 h. The disappearance of each hydroxycinnamic acid was confirmed by thin layer chromatography (mobile phase: n-hexane:ethyl acetate, 2:1, v:v), then the remaining solvent was evaporated under reduced pressure by a rotary evaporator. Benzene was added to the residue, and then, the solvent was again evaporated by a rotary evaporator to obtain a crude product. The resulting crude product was separated and purified by silica gel column chromatography (diameter=5.5×7.0 cm; 60 g; mobile phase: n-hexane:ethyl acetate 2:1, v:v) to obtain the corresponding methyl hydroxycinnamates. Each product was recrystallized in n-hexane to obtain white crystals (5.09 g, 98%) with a purity higher than 95%.
2.8 Recombinant enzymes
Recombinant enzymes from A. niger were produced and purified from culture media as described previously for feruloyl esterase A (rAnFaeA) (Record, Asther et al., 2003) and B (rAnFaeB) (Levasseur et al., 2004), respectively.
2.9 Enzyme assays
Feruloyl esterase activity was determined as described previously (Ramirez, Arrizon et al., 2008), using different methyl hydroxycinnamates as substrates: Methyl ferulate (MF), methyl sinapinate (MSA), methyl caffeate (MC) and methyl p-coumarate (MpCA). Each reaction mixture contained 10 μL of the corresponding methyl hydroxycinnamate (50 mM) with p-nitrophenol (5 mM), previously dissolved in tert-butanol, 90 μL of MOPS (2.5 mM) at pH 7.2, and 20 μL of an appropriately diluted enzyme solution.
Acetyl esterase activity was assayed by measuring the amount of p-nitrophenol released from p- nitrophenyl acetate (pNPA) or p-nitrophenyl butyrate (pNPB), based on the method of (Biely et al., 1985). The reaction mixture contained 10 μL of pNPA (10 mM) previously dissolved in tert-butanol, 90 μL of MOPS (50 mM) at pH 7.2, and 20 μL of appropriately diluted enzyme solution.
The absorbance (Abs) of p-nitrophenol was recorded at 410 nm every 30 s during 30 min in a microplate reader (x-Mark, Bio-Rad) and the slope (Abs min−1) was employed to calculate the initial rate of the reaction. Standard curves in the presence of each hydroxycinnamic acid or p-nitrophenolate were performed in the assay conditions to quantify the feruloyl and acetyl esterase activity, respectively. One Unit (U) of esterase activity was defined as the amount of enzyme that catalyses the release of 1 μmol of hydroxycinnamic acid or p-nitrophenolate per min at 35 °C under the assay conditions. Activities were measured at least in triplicate using blanks without enzyme to take into account spontaneous hydrolysis.
2.10 Zymographic analysis
Zymograms were performed based on a previous report (Singh et al., 2006) for lipase activity and adapted for feruloyl or acetylxylan esterases. A native polyacrylamide gel electrophoresis (PAGE) was carried out using 12% polyacrylamide gel. Proteins were migrated at 100 mV in a gel casting apparatus (Mini-PROTEAN® system, Bio-Rad) using Tris- Glycine buffer (Tris 25 mM, Glycine 200 mM, pH 8.3) at 4 °C. The gels were rinsed three times with distilled water and equilibrated in MOPS 2.5 mM (pH 7.2) for 30 min at room temperature. After that, the native gels were submerged in a solution containing each hydroxycinnamic ester 5 mM or pNPA 1 mM and phenol red 0.02% in a MOPS 2.5 mM (pH 7.2) buffer and incubated at 30 °C. Depending on the amount of esterase, the activity was observed within 5-15 min of incubation as a yellow band over a pink background.
2.11 Isolation and identification of acetylxylan esterase
Protein isolation was done by electrophoretic elution from polyacrylamide gel based on (Sá-Pereira et al., 2000). A preparative zymogram of a crude enzyme sample obtained after 72 h of culture of A. niger PCS6 grown in corn bran was done. The gel was revealed with MF and a band corresponding to an active enzyme with relative mobility (Rf) of 0.7 was cut vertically along the gel. The cut band was subsequently minced, introduced into a glass tube with frit and placed in an Electro-Eluter apparatus (Model 422, Bio-Rad). Electro-elution was carried out as recommended by the supplier, using a 3 kDa membrane in a Tris-Glycine buffer (Tris 25 mM, Glycine 200 mM, pH 8.3) at 4 °C. Immediately the protein retained by the membrane was recovered and conserved at 4 °C to perform the enzymatic assays and SDS-PAGE analysis. The purity of enzyme was checked by SDS-PAGE at 12% revealed by silver staining (ProteoSilver T M Silver Stain Kit, Sigma). To determine the N-terminal sequence of the AcXE from PCS6, the protein was first separated by means of the SDS-PAGE procedure using a 12% acrylamide gel and then electroblotted onto polyvinylidenedifluoride membrane (PVDF, Bio-Rad) with a Tris-borate buffer (Tris 50 mM, 50 mM borate, pH 8.3). Immediately, the membrane was colored with Ponceau red; the band observed at 32 kDa was cut and subjected to analysis on an Applied Biosystem Model 476 A gas- phase sequencer. Finally, a search in the EMBL, GenBank, Swiss-Prot protein sequence data bases was made resulting in a 100% identity with the N- terminal amino acid sequence with accession number GenBank/NCBI: EHA25274.1/XP 001395572.
3 Results and discussion
3.1 Phenolic acid characterization of agro-industrial by-products
It has been shown that the mechanism of induction of certain kind of FAEs involve phenolic acids (de Vries & Visser, 1999; de Vries, vanKuyk et al., 2002). In this sense, it's very important to know the availability, amount and kind of ester-bounded phenolic acids present in agro-industrial by-products. The content of total, free or esterified ferulic, p-coumaric, and caffeic acid in coffee pulp, wheat and corn bran are shown in Table 1. In all cases ester linked phenolic acids, which may be hydrolyzed by feruloyl esterases, were the most abundant (>63%, w w−1). In coffee pulp, the three phenolic acids were clearly identified, with esterified caffeic acid (hydroxylated) being the most abundant (48%, w w−1). Meanwhile, in the case of wheat and corn bran, caffeic acid was not detected and esterified ferulic acid (methoxylated) was present in a high proportion (about 88%, w w−1). Furthermore, the concentration of esterified ferulic acid in corn bran (5.7 mg g−1) was 3 times higher than in wheat bran. These results agree with the report of Benoit et al. (2006), who demonstrated that coffee pulp is a by-product rich in caffeic acid, whereas corn bran had a higher content of ferulic and p-coumaric acids. Nevertheless, in our work the highest amount of caffeic acid was found as chlorogenic acid (2.4 mg g−1) and consequently removed during the free phenolic acids analysis, still the total amount of caffeic acid in coffee pulp is around 63% of that reported by Benoit et al. (2006). On the other hand, the same authors found 3 to 5 times more ferulic and p-coumaric acid in corn bran, probably because of the different source of the raw material.
3.2 Carbohydrate esterase activity production with various agro-industrial by-products
There are many reports related to the use of agro- industrial by-products as substrates for enzymes production.
In particular, the carbohydrate esterases FAEs and AcXEs are typically produced using wheat bran, sugar beet pulp and sugarcane bagasse (Christov & Prior, 1993; Topakas et al., 2007). However, there are no report related to the production of AcXEs using corn bran or coffee pulp and only a few reports related to FAEs production using these by-products as substrate (Shin & Chen, 2006; Ramirez, Arrizon et al., 2008; Kumar et al., 2011; Ou et al., 2011; Pérez-Morales et al., 2011; Torres-Mancera et al., 2011). Moreover, it is unclear what kind of these carbohydrate esterase encoding-genes is expressed using these by-products as a carbon source. Indeed, only one enzymatic substrate (ferulic acid methyl ester or acetylated xylan) has been used in all previous studies to measure the global FAEs or AcXEs activity. Because FAEs and AcXEs have different substrate specificities it is necessary to determine the enzymatic activity using different selective substrates. MFA and MCA can be used to differentiate between AnFaeA and AnFaeB (Benoit, Navarro et al., 2006), while pNPA allows FAEs and AcXEs to distinguish between (Ghatora et al., 2006).
In order to evaluate the effect of different local agro-industrial by-products for the selective induction of FAEs and AcXEs, A. niger PCS6 was grown on corn bran, coffee pulp and wheat bran as the main carbon source substrate in SSF and compared with a control using only glucose as the carbon source. In general, for all by-products used, enzyme production profiles in the time course of SSF followed the same pattern, while using glucose as the sole carbon source, only a basal esterase activity was found (Figure 1). The esterase activities increased in the early stages of growth, reaching their highest level after two days of culture and maintained without significant changes during the next three days. Esterase activity measured on both methyl esters (MF and MC) was higher for cultures grown on corn bran (Figure 1a and Figure 1b, respectively), where the activity on MF was 5.27 U g−1 of dry matter; being 5 and 25 times more than those grown on wheat bran and coffee pulp, respectively. Moreover, the activity measured on MC was 0.66 U g−1 of dry matter, being 1.6 and 1.5 times more than those found using wheat bran and coffee pulp, respectively. These results suggest that corn bran is suitable for a high production of both AnFaeA and AnFaeB activities. It is noteworthy that these titles of activity are high compared to those reported in literature for cultures of Aspergillus niger by solid state fermentation. Asther et al. (2002) found 5 nkat g -1 of dry matter (0.3 U g-1 of dry matter measured on MF) in cultures of A. niger grown on sugar beet pulp. Besides, Donaghy et al. (1995) found 4.5 y 0.9 mU g−1 of dry matter (measured in MF) in cultures of A. niger grown on wheat bran and sugar beet pulp, respectively.

Fig. 1 Effect of carbon source on esterase production. Activities were measured with methyl ferulate (A), methyl caffeate (B), and p-nitrophenyl acetate (C) as substrates. Symbols: culture supernatant of A. niger PCS6 growthin corn bran (•), coffee pulp (o), wheat bran (▼) and glucose (∆).
On the other hand, activity measured on pNPA was higher for cultures grown on wheat bran (activity was 50.31 U g−1 of dry matter), followed by cultures grown on corn bran (33.4 U g−1 of dry matter) and coffee pulp (23.3 U g−1 of dry matter) (Figure 1c), suggesting the production of acetylxylan esterases. However, this enzymatic activity could be attributed to other enzymes capable of hydrolyzing pNPA, such as some lipases and other esterases (Chahinian & Sarda, 2009). Although enzyme activity measurement can be a strong indication of the selective production of enzyme depending on each by-product, it is necessary to verify whether the activity detected corresponds to one or more enzymes produced during fermentation as shown below.
3.3 Analysis of carbohydrate esterase production by zymogram
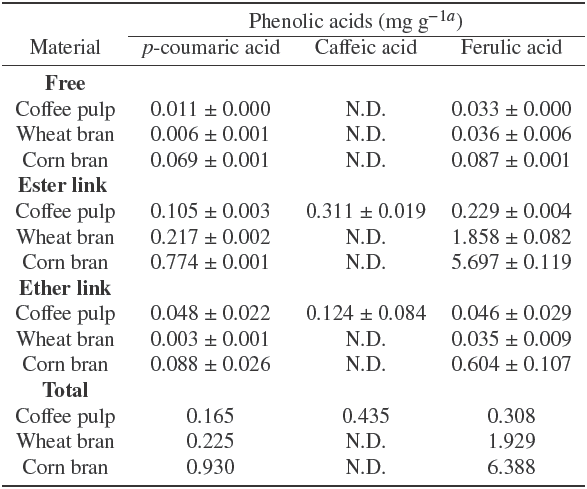
For a better understanding of esterases induction in SSF using the by-products prior described, zymographic assays in native polyacrylamide gel electrophoresis (PAGE) were implemented (Figure 2). Purified recombinant feruloyl esterases from A. niger (rAnFaeA and rAnFaeB) were used as reference enzymes (line 1 and 2, respectively). Zymograms were stained with phenol red and incubated in MF (Figure 2a), MC (Figure 2b), and pNPA (Figure 2c), until yellow bands corresponding to esterase activity appeared. As expected, the band corresponding to rAnFaeA was observed only in the zymogram incubated with MF at a relative mobility (R f ) value of 0.79; while the rAnFaeB band appeared in both zymograms (incubated with MF and MC) at a Rf value of 0.13, showing a more intense band with MC. Both FAEs were observed in the zymogram incubated with pNPA, but only when ten times more enzyme was loaded (lines 6 and 7). In order to compare the induction and kind of esterase activities using all by- products tested, equal volumes of the culture broths obtained after 72 h of solid fermentation were loaded into the gels (lines 3 to 5). The zymographic assays confirmed the findings observed during the kinetics of FaeA, FaeB and AcXE activity production (Figure 1). However, a band with a R f value of 0.7 (Figure 2a, b and c; lanes 3 -5), later identified as an A. niger acetyl xylan esterase A (AcXEA) (see section 3.4 below), was observed in all gels. The major production of AcXEA was obtained when wheat bran was used as SSF substrate (Figure 2c, lane 5). Nevertheless, AcXEA activity could also be detected in zymograms incubated with MF and MC, but with scarcer intensity than that incubated with pNPA (Figure 2a and Figure 2b, lanes 3-5).
Fig. 2 Zymogram analysis of esterases detected with methyl ferulate (A), methyl caffeate (B), and p-nitrophenyl acetate (C) as substrates. Samples were taken at 72 h of culture. The same volume of each culture supernatant was applied to zymogram. Lanes: 1 and 2, rAnFaeA and rAnFaeB; respectively; lanes 3-5, culture supernatant of A. niger PCS6 growth in corn bran, coffee pulp and wheat rAnFaeB, respectively.
These results suggest that corn bran is a suitable inducer for all three esterases, with a major production of feruloyl esterase activity; while wheat bran and coffee pulp could be used for the preferential AcXEA and AnFaeB activity production, respectively.
The mechanism of induction of A. niger FAEs using methoxylated and hydroxylated hydroxycinnamates as substrates, has been previously described (de Vries & Visser, 1999; de Vries, vanKuyk et al., 2002). These authors observed that the transcription of FaeA gene occurred in the presence of methoxylated aromatic compounds, such as ferulic acid and sinapinic acids, while highest levels of FaeB gene transcription occurred in the presence of hydroxylated compounds, e.g. caffeic acid and p- coumaric acids. Thus, the induction of the different FAEs type could be attributed to the proportion of hydroxycinnamates present in the by-products used as substrates. The high concentration of ferulic acid in corn bran, suggests its potential as an inducer of type A and D feruloyl esterases (e.g. A. niger feruloyl esterase A), while the high concentration of hydroxylated phenolic acids in coffee pulp (i.e. caffeic and p-coumaric acid) suggests its potential as an inducer of A. niger feruloyl esterase B (type C).
Moreover, it has been observed that the production of AcXEs by filamentous fungi is related to the degree of acetylation of the carbon source employed (Navarrete et al., 2012). It has been reported that corn bran and wheat bran are hemicellulosic biomasses with a high degree of acetylation (Agger et al., 2010), which may explain why these by-products are good substrates for the production of AcXEs.
3.4 Acetylxylan esterase identification
Zymographic analyzes conducted for crop samples revealed the presence of an enzyme very active on pNPA, capable to hydrolyze methyl esters. This enzyme showed a different activity profile than the recombinant FAEs of A. niger used as reference enzymes. In order to identify this protein, a direct purification was carried out from a polyacrylamide gel. For this purpose, a preparative zymographic electrophoresis of the crude enzyme preparation obtained from corn bran solid fermentation was done. After that, the corresponding band (R f value of 0.7) was cut along the gel and the protein electro-eluted at 4 °C in order to retain the esterase activity.
The electro-eluted purity of the sample was analyzed by SDS-PAGE (Figure 3). The major protein band was observed around 32 kDa and then transferred into a PVDF membrane. Linden et. al. (1994) isolated an acetyl esterase from A. niger whose molecular weight elucidated by SDS-PAGE analysis was around from 32 kDa.

Fig. 3 Silver stained SDS-PAGE of partially purified AcXEA. Lanes: 1, molecular weight markers; 2, AcXEA electro-eluted.
The 25 amino acids sequence of the N-terminal of the protein was obtained (SGSLQQITDFGDNPTGVXMYIYXXN) and after a computer search in the EMBL, GenBank, Swiss- Prot protein sequence data bases, revealed 100% identity with the N-terminal amino acid sequence of acetyl xylan esterase A from A. niger (GenBank/NCBI accession number: EHA25274.1/XP_001395572).
The specific activity of identified AcXEA on various substrates is shown in Table 2 which is compared to those obtained for rAnFaeA and rAnFaeB using the same methodology. The electro-eluted esterase showed a higher activity on pNPA than in pNPB (about 10 times), as expected for an AcXE (Ghatora, Chadha et al., 2006). Interestingly, the electroeluted AcXEA had activity on methyl hydroxycinnamates, however, the specific activity was lower in comparison with pNP esters; a similar specificity for all the methyl esters with exception of methyl caffeate in which the specific activity, was about one half of other methyl esters showing the versatility of this kind of enzymes to accept several substrates. In contrast, rAnFaeA and rAnFaeB are more selective enzymes on the hydrolysis of methyl hydroxycinnamates than pNP esters; rAnFaeA showed a high preference of hydrolysis of methyl sinapate and ferulate (about 12 and 5 times more in comparison of pNPB); rAnFaeB showed a high preference of hydrolysis for the other two complementary hydroxycinnamates, methyl p- coumarate and caffeate (about 100 and 60 times more in comparison of pNP esters).
Table 2 Substrate specificity of AcXEA a compared to recombinant feruloyl esterases (rAnFaeA and rAnFaeB).
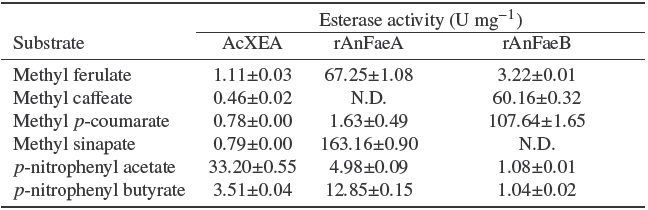
a AcXEA was partially purified by electroelution from a zymogram band. Activity values are expressed as U mg-1 protein. N.D.: activity not detected. Results are mean values of two or three determinations. One unit (U) is defined as the amount the enzyme that catalyses the release of 1 µmol of acid per minute.
3.5 Corn bran for high production of feruloyl and acetylxylan esterases
As described previously, the production of A. niger AcXEA activity using corn bran as substrate was close to that produced with wheat bran (about 0.65 in pNPA activity), where the AcXEA activity can be attributed to acetylation degree of each by-product. In other hand, the high content and availability (free and ester link) of ferulic and p-coumaric acid in corn bran (Table 1) promotes a high production of A. niger feruloyl (AnFaeA and AnFaeB) esterases, with a global feruloyl activity about 5 and 1.5 times with methyl ferulate and caffeate (for AnFaeA and AnFaeB, respectively), in comparison with the closest by-product. These results suggest that corn bran is an excellent substrate for the high production of these kind of fungal carbohydrate esterases by SSF. Work is ongoing in order to use corn bran as substrate in other Aspergillus species to increase their feruloyl and acetylxylan esterase production by SSF.











 nueva página del texto (beta)
nueva página del texto (beta)