Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 no.3 Ciudad de México Dez. 2012
Biotecnología
Advances in the phytochemistry of Cuphea aequipetala, C. aequipetala var. hispida and C. lanceolata: Extraction and quantification of phenolic compounds and antioxidant activity
Avances en la fitoquímica de Cuphea aequipetala, C. aequipetala var. hispida y C. lanceolata: Extracción y cuantificación de los compuestos fenólicos y actividad antioxidante
B.A. Cardenas-Sandoval, A.R. López-Laredo, B.P. Martínez-Bonfil, K. Bermúdez-Torres and G. Trejo-Tapia*
Departamento de Biotecnología, Centro de Desarrollo de Productos Bióticos, Instituto Politécnico Nacional, P O. Box 24, CP 62730, Yautepec, Morelos, México. *Corresponding author. E-mail: gttapia@ipn.mx
Received 25 of June 2012
Accepted 4 of October 2012
Abstract
Cuphea aequipetala and Cuphea lanceolata native to Mexico are used in folk medicine. Extraction procedure standardization was performed and the amount of total phenolic compounds and flavonoids was determined in methanol extracts (obtained by stirring for 24 h) from various organs of C. aequipetala, C. aequipetala var. hispida and C. lanceolata. The antioxidant properties of extracts were compared using in vitro free radical-scavenging assays (1,1-diphenyl-2-picrylhydrazyl (DPPH•+) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS•+)) and the reducing power of phosphomolybdenum (PPM). A significant correlation was found between antioxidant activity and the amount of antioxidant components. Flowers of C. lanceolata showed the highest concentration of phenolic compounds (62.79±0.05 mg gallic acid equivalfnts (GAE)/g dry weight (DW) and the highest content of flavonoids was found in leaves of C. aequipetala (196.83±2.9 mg quercetin equivalents (QE)/g DW). The highest free radical-scavenging fctivity against DPPH•+ was found in -eaves of C. aeqrnpetala var. hispida (173.33±2.12 μmol trolox/g DW), for ABTS- in flowers ol C. aequipetala (541.10±2.32 μmol trolox/g DW) and for 5PM in leaves oS C. aequipetala (1186.25±3.17 μmol trolox/g DW). Qualitative analysis indicated the presence of the flavonoid quercetin 3-β-D-glucoside in all the species of Cuphea amongst other less polar flavonoids in C. aequipetala var. hispida. Cuphea spp. are prospective sources of phenolic compounds.
Keywords: antioxidant activity, Cuphea, phenolic compounds, free radical-scavenging, reducing power.
Resumen
Cuphea aequipetala y Cuphea tanceolata son especies nativas de México utilizadas en medicina iradicional. Se estandarizó el procedimiento para obtener extractos y se determinó, en extractos metanólicos (obtenidos en agitación por 24 h), el contenido de compuestos fenólicos y flavonoides totales de varios órganos de C. aequipetala, C. aequipetala var. hispida y C. lanceolata. Sus propiedades antioxidantes fueron comparadas usando métodos in vitro (DPPH•+ y ABTS•+) y el de poder reductor del fosfomolibdeno. La concentración mas alta de compuestos fenólicos se presentó en las flores de C. lanceolata (62.79±0.06 mg equivalentes de ácido gálico (EAG)/g pesos seco (PS); mientras que la de flavonoides en las hojas de C. aequipetala (196.83±2.9 mg equivalentes de quercetina (EQ)/g PS). Las hojas de C. aequipetala var. hispida presentaron la actividad de captura de radicales libres DPPH (173.33±2.12 μmol trolox/g PS), las flores de C. aequipetala la de captura de radicales libres ABTS (541.10±2.32 μmol trolox/g PS) mientras que el poder reductor más alto se observó en las hojas de C. aequipetala (1186.25±3.17 μmol trolox/g PS). Se encontró una correlación positiva significativa entre la actividad antioxidante y la concentración de compuestos antioxidantes. El análisis químico cualitativo mediante TLC indicó la prescencia del flavonoide quercetina 3-3-D-glucosido en todas las especies de Cuphea y de otros flavonoides menos polares en C. aequipetala var. hispida. Cuphea spp. es una fuente natural de compuestos fenólicos.
Palabras clave: actividad antioxidante, Cuphea, compuestos fenólicos, captura de radicales libres, poder reductor.
1 Introduction
The genus Cuphea (Lythraceae) comprises > 260 species native to the Americas distributed from Mexico to Brazil. Cuphea species are cultivated as sources of oils rich in medium-chain fatty acids (Graham and Kleiman, 1992; Phippen, 2010; Tisserat et al., 2012) and are used in traditional ("folk") medicine for their antioxidant (Schuldt et al., 2004), antihypertensive (Braga et al., 2000), cytotoxic (Wang et al., 1999), antiprotozoal (Barbosa et al., 2007) and hypocholesterolemic activities (Biavatti et al., 2004). For instance, the leaves of C. carthagenensis represent a significant source of phenolic antioxidants that may have potentially beneficial cardiovascular effects (Schuldt et al., 2004). Cuphea aequipetala Cav., "hierba del cancer" (Spanish) or "Tozancuitlacxolli" (Nahuatl), is native to Mexico and grows in open and humid fields of pine-oak woods 2000-2540 m above sea level (m asl) (Graham, 1991). Infusion or poultice of the aerial parts (leaf and stem) of the plant is used in Mexican folk medicine to treat tumors, pounds and wounds (Aguilar-Rodriguez et al., 2012). Aqueous extracts of the aerial parts showed strong activity against Helicobacter pylori (Castillo-Juarez et al., 2009), whereas organic extracts of flowers, leaves and stems showed cytotoxic activity against carcinoma cells of the human larynx (HEp-2 cell line) (Waizel-Bucay et al., 2003) and acetone-water extracts of the whole plant showed activity against carcinoma cells of human prostate (Vega-Avila et al., 2004). Cuphea lanceolata W.T. Aiton, "Atlanchana" or "Atlancan" (Spanish) and "cigar plant" (English) is also native to Mexico. Infusion of the aerial part is recommended in folk medicine as an anti-diarrheal agent (Waizel, 2006). It grows in open fields of dry tropical forests at 2250-2500 m asl (Graham, 1991). This species has been included in breeding programs to domesticate Cuphea. The species of the genus Cuphea produce high amounts of medium chains fatty acids, which represent an alternative to coconut oil in soaps, detergents and other products or as an antimicrobial pesticide in commercial food handling (Millam et al., 1997; Phippen, 2010). Profiles of fatty acid are species specific (Wolf et al., 1983; Phippen et al., 2006), showing that within the genus Cuphea exists a high genetic variation.
There is growing interest in the use of medicinal plants as sources of natural antioxidants as potentially side effect-free alternatives to synthetic compounds (Juntachote and Berghofer, 2005; Krishnaiah et al., 2010) and as adjuvants in cancer therapy (Saxena et al., 2010). Antioxidants, including phenolic compounds (e.g., phenolic acids, flavonoids, tannins) have diverse biological effects, such as antiinflammatory, cytotoxic and anti-tumor effects, as a result of their antioxidant activity (Vega, 2005; Krishnaiah et al., 2010). Quantity and quality of plant phenolics vary in response to environmental factors, such as light intensity and nutrient availability, but also on different genetic levels (between and within species and clones) and between different physiological and developmental stages (Klepacka et al., 2011; Estrada-Zúñiga et al., 2012; Naghiloo et al., 2012). Conversely, the activity of antioxidants may be influenced by extract preparation (Perez-Jimenez et al., 2008; Jimenez et al., 2011; Garcia-Marquez et al., 2012). Increasing interest has been focused into the procedures for extract preparation because of their influence in the yield and modification of the activity of compounds (Bolling et al., 2009; Turkmen et al., 2006).
Here, we report the standardization of the extraction procedure, the determination of the concentration of total phenolic compounds and flavonoids of various organs of wild-growing C. aequipetala, C. aequipetala var. hispida and C. lanceolata. Furthermore, the antioxidant capacity of those extracts was shown to correlate well with phytochemical content.
2 Materials and methods
2.1 Plant material
Cuphea aequipetala and C. aequipetala var. hispida were collected at the flowering stage, in order to standardize phenological stage of plants, in Lagunas de Zempoala (State of Mexico, Mexico) at 2860 m asl (latitude 19° 02' N, longitude 99° 19' W), both species grow mixed as open ground layer. C. lanceolata plants were collected at the flowering stage in Yautepec (State of Morelos, Mexico) at 1267 m asl (latitude 18° 53', longitude 99° 04' W) as secondary vegetation. Plants (25-30) were excised into roots, stems, leaves and flowers. They were dried at room temperature under shade for 1 week. Dry material was ground manually into a fine powder (particle size < 250 μm) using a pestle and mortar. Plants were positively identified as C. aequipetala Cav. (voucher numbers 21181 and 21172), C. aequipetala Cav. var. hispida Koehne (voucher numbers 21170 and 21171) and C. lanceolata W.T. Aiton (voucher numbers 13239, 13240 and 13238) at the Herbarium of the Universidad Autonoma del Estado de Morelos (UAEM).
2.2 Standardization of the extraction procedure
Four different methods were tested to evaluate the efficacy of the extraction type of antioxidant compounds. For these evaluations, 100 mg of the powdered dried leaves of C. aequipetala were used. Following extraction methods were evaluated: 1) sequentially extraction: 100 mg of the homogenized samples were extracted with 50 mL of hexane under agitation for 24 h at room temperature, filtered through a Whatman No. 1 filter paper (Whatman, Maidstone, UK) and the supernatant collected. The pulp residues were re-extracted by the addition of 50 mL of ethyl acetate under agitation for 24 h at room temperature, filtered through a Whatman No. 1 filter paper and the supernatant collected. The pulp residues were re-extracted by the addition of 50 mL of methanol under agitation for 24 h at room temperature, filtered through a Whatman No. 1 filter paper and the supernatant collected. All supernatants were concentrated to dryness in a rotary evaporator (Büchi-490; Büchi, Switzerland). The collected supernatants were analyzed separately; 2) extraction with methanol (50 mL) under stirring for 24 h; 3) extraction with methanol (50 mL) on a water bath at 60°C for 30 min; 4) aqueous extraction (50 mL) of freshly boiled distilled water and 30 min rest and filtering through Whatman number 1 paper and rapidly cooled under tap water. All extracts were filtered and evaporated to dryness in a rotary evaporator and stored at -70°C in the dark until analyses. Methanol extracts were evaporated at 210 mbar and 40°C; aqueous extracts at 50 mbar and 60°C. For the analysis, extracts were resuspended in 1 mL of their respective solvent. Extract yields were calculated according to the following: Extraction yield (%) = [weight of the freeze-dried extract/weight of the original sample] x 100, and were expressed as milligrams of extract per g (dry weight; DW) of leaves. Extractions were performed in triplicate. Yields of total phenols, total flavonoids, and antioxidant activity were evaluated to determine the best extraction method, which was followed for all dried organs from Cuphea spp.
2.3 Determination of total phenolic-compound content
Phenolic compounds in methanol and aqueous extracts were estimated using the Folin-Ciocalteu colorimetric method (Shohael et al., 2006). Each extract (100 juL) was mixed with 2.5 mL of deionized water, and 100 UL of Folin-Ciocalteu reagent added. The mixture was incubated at room temperature for 6 min before an aqueous solution of sodium carbonate (0.5 mL, 20%, w/v) was added, and the mixture gently mixed. A blank sample was prepared by mixing 100 uL methanol with the reagents. After 30 min, the color was fully developed and the absorbance measured at λ=760 nm. The total phenolic-compound content was determined using a standard curve prepared with gallic acid (0-25 ug/mL). Results were expressed as milligrams of gallic acid equivalent (GAE) per gram of dry weight (DW). Samples were analyzed in triplicate.
2.4 Determination of total flavonoid content
The total flavonoid content was determined using a colorimetric assay as described by Shohael et al. (2006). Each extract (250 μL) was mixed with 1.25 mL of de-ionized water, and 75 μL of an aqueous solution of NaNO2 (5%, w/v) added. The mixture was thoroughly vortex-mixed and incubated at room temperature for 6 min. Then, 150 μL of an aqueous solution of AlCl3 (10%, w/v) were added. After a further 5 min, 0.5 mL of an aqueous solution of NaOH (1 M), and 2.5 mL of de-ionized water, were added. Finally, the mixture was incubated 30 min at room temperature and the absorbance measured at 510 nm using methanol as a blank sample. Quercetin was used to create a calibration curve (0-150 μg/mL). The total flavonoid content was expressed as milligrams of quercetin equivalent (QE) per gram of DW. Samples were analyzed in triplicate.
2.5 DPPH•+
The free radical-scavenging activity of extracts was quantified by spectrophotometric means using a DPPH•+assay (Sanchez-Moreno et al., 1998). A stock solution of freshly prepared DPPH•+(3.9 mL, 60 μM) was mixed with 100 μL of each sample extract at 5 mg/mL dissolved in methanol. The mixture was shaken vigorously and incubated for 6 min at room temperature in the dark. The absorbance was immediately recorded at 515 nm. Trolox (0-15 μmol/L) was used as a reference standard. Results were expressed as micromoles of trolox per gram of DW based on a calibration curve (R2 = 0.993). The assay was carried out in triplicate.
2.6 ABTS•+
An ABTS radical-scavenging assay was carried out using the improved (ABTS•+) method described by Re et al. (1999) with slight modification. Briefly, the ABTS•+ radical cation was generated by the reaction of 7 mmol/L ABTS•+ and 2.45 mmol/L potassium persulfate for 16 h at room temperature in the dark. ABTS•+solution was diluted with methanol to an absorbance of 0.7±0.05 at 734 nm. Each extract (50 μL) was mixed with 1.9 mL of ABTS•+solution. The mixture was incubated for 6 min at room temperature in the dark and the absorbance recorded immediately at 734 nm. Trolox solution (final concentration, 0-15 μmol/L) was used as a reference standard. The results were expressed as micromoles of trolox per gram of DW based on a calibration curve (R2 = 0.975). The assay was carried out in triplicate.
2.7 PPM
The reducing power assay using PPM has been described by Prieto et al. (1999) and is based upon the reduction of molybdenum (Mo) (VI) to Mo (V) by antioxidant compounds and the formation of a green Mo complex with maximum absorption at 695 nm. The assay can be used to detect antioxidants such as ascorbic acid, phenolic compounds, and carotenoids. Each extract (100 μL; final concentration, 5 mg/mL) was incubated at 95°C with the reagent solution (sodium phosphate (28 mM) and ammonium molybdate (4 mM) in sulfuric acid 0.6 M) for 90 min. The mixture was allowed to stand at room temperature for 30 min and the absorbance recorded. Trolox solution (final concentration, 0-80 μmol/L) was used as a standard. Results were expressed as micromoles of trolox per gram of DW (Diouf et al., 2009) based on a calibration curve (R2 = 0.9974). The assay was conducted in triplicate.
2.8 TLC analysis
An aliquot (15 μL) of plant extract (5 mg mL-1) was carefully spotted on a 5x5 cm TLC layer (silica gel 60 F254; Merck) which was then developed with a ethyl acetate:methanol:H2O (76:16:8, v:v) solvent system. The silica plate was dried and placed upside down for 2-3 min in a 0.01 mM DPPH solution in methanol (Lopez-Laredo et al., 2012). Stained silica layer revealed a purple background with yellow spots corresponding to the resolved bands with radical scavenger capacity. TLC plates were inspected also under UV light at 254 and 365 nm. Another set of TLC plates was sprayed with PEG (5%, v/v) before staining with a methanolic solution of diphenylboric acid-β-ethylamino ester (1%, p/v). Retardation factor (Rf) is a relative measure of the substance position in the chromatogram with respect to the position of the solvent front. It is the most widely used descriptor of position in TLC and was calculated according to the following equation: RF = zi/(zi - z0), where zi is the migration distance of substance, zf is the migration distance of front measured from the immersion line, and z0 is distance between immersion line and sample application (Reich and Schibli, 2007).
2.9 Statistical analyses
Statistical analyses were performed by two-way ANOVA. The all-pairwise multiple comparison procedure of Duncan was used to determine statistically different values at P < 0.05. Pearson correlation coefficients and P values were used to show correlations and significance at P < 0.05. SigmaPlot for Windows version 11.0 (Systat Software Inc., San Jose, CA, USA) was used to carry out statistical analyses.
3 Results and discussion 3.1
Standardization of extraction procedures
Extraction yields, total content of phenolic compounds, total flavonoids, and antioxidant activity were evaluated to determine the best extraction method (Table 1). The amount of extractable components ranged from 30 mg/g DW (hexane extract) to 324 mg/g DW (methanol in a water bath). The amount of total phenol compounds increased in the order methanol/water bath > methanol/stirring > methanol/sequential extraction > water extract > ethyl acetate > hexane (P < 0.05) (Table 1). All fractions were rich in flavonoids except ethyl acetate and hexane fractions, and the highest concentration was observed in the methanol/stirring method (196.14±2.93 mg QE/g DW, P < 0.05).
This extraction procedure allowed obtaining the highest antioxidant activity (free-radical scavenging activity and phosphomolybdenum reducing power). Aqueous extract presented a similar content of phenolic compounds (38.17±0.41 mg GAE/g DW) but a higher content of flavonoids (143.32±1.98 mg QE/g DW) than that of the methanol fraction result of the sequential extraction. Free-radical scavenging of the aqueous extract (411.38± 1.9 jumol trolox/g DW and 654.06±37.2 μmol trolox/g DW) and reducing power (2759.7±25.6 μmol trolox/g DW) was less than that of the methanol extracts regardless of the procedure followed for their preparation. Hexane fraction did not present antioxidant activity whereas ethyl acetate fraction presented low free-radical scavenging activity against DPPH and ABTS (24.33±1.4 and 47.72±0.8 μmol trolox/g DW, respectively). Variations in the quantity of total phenolic compounds and flavonoids in the extracts with different solvents and extraction methods have been reported. For instance, in the methanol extracts of Lythrum salicaria (Lythraceae), the content of phenolic compounds and flavonoids was lower than that in water extracts (Tunalier et al., 2007). In contrast, the content of phenolic compounds in methanol and water extracts of Lawsonia inermis (Lythraceae) was similar (Guha et al., 2011). The amount of extracted phenolic compounds and/or flavonoids depends on the temperature and time extraction, but also on the polarity of used solvents and on the species/organ specific content of less or more polar compounds (Serrano-Maldonado et al., 2011; Garcia-Marquez et al., 2012). In the present study, the analyses shown below were undertaken by extraction using methanol and stirring for 24 h due to significantly higher values of phenols, flavonoids and antioxidant activity.
3.2 Total phenolic compounds and flavonoids content
In general, phenolic compounds are responsible for many interactions between plants and their biotic and abiotic environment. These compounds have an organ specific distribution and accumulate differentially during ontogenesis and under the influence of environmental factors (Hutzler et al., 1998; Ayan et al., 2007). Moreover, phenolic compounds biosynthesis is subcellularly compartmentalized and the role of cell compartments has not been totally elucidated. For instance, L-phenylalanine (precursor), quercetin and kaempferol glycosides (flavonoids) are synthesized in the chloroplasts but other cell compartments such as cytosol, vacuole or endoplasmic reticulum are involved in the biosynthesis of precursors, intermediates and end products (Santiago et al., 2000; Zhao and Dixon, 2010). At this level, they fulfill three major functions: substrates, energy sources and regulators (Kefeli et al., 2003). In the aerial parts phenolic compounds play a role as part of the defense system of plants against pest and pathogens (Lattanzio et al., 2006) but also as pigments to attract pollinators (Buer et al., 2010). Furthermore, in roots flavonoids are involved as signal molecules in the symbiosis plant-bacteria (Buer et al., 2010).
References show that organ specific accumulation of phenolic compounds is species specific (Bhatt et al., 2012; Liu et al., 2012). For the genus Cuphea, seems to be the aerial parts where the higher levels of phenolic compounds are found (Calzada, 2005; Krepsky et al., 2012). Results of present work show that the highest content of total phenolic compounds was found in the leaves of C. aequipetala and C. aequipetala var. hispida (55.62±0.50 and 60.74±0.23 mg GAE/g DW, respectively), while for C. lanceolata flowers showed the highest amount of these compounds (62.79 ±0.05 mg GAE/g DW) (Fig. 1A). In the three species, the stem contained the lowest level of phenolic compounds. Similarly, flavonoids were more abundant in the leaves of C. aequipetala and C. aequipetala var. hispida (196.83±2.94 and 124.74±1.28 mg QE/g DW, respectively) and in the flowers of C. lanceolata (135.81 ±1.55 mg QE/g DW) (Fig. 1B). According to statistical analyses, the total content of phenolic compounds was specific to species (P < 0.05), variety (P < 0.05), and plant organ (roots, stems, leaves or flowers, P < 0.05). For total phenolic compounds, flavonoid concentration was highly dependent upon the species (P < 0.05), variety (P < 0.05), and plant organ (P < 0.05).
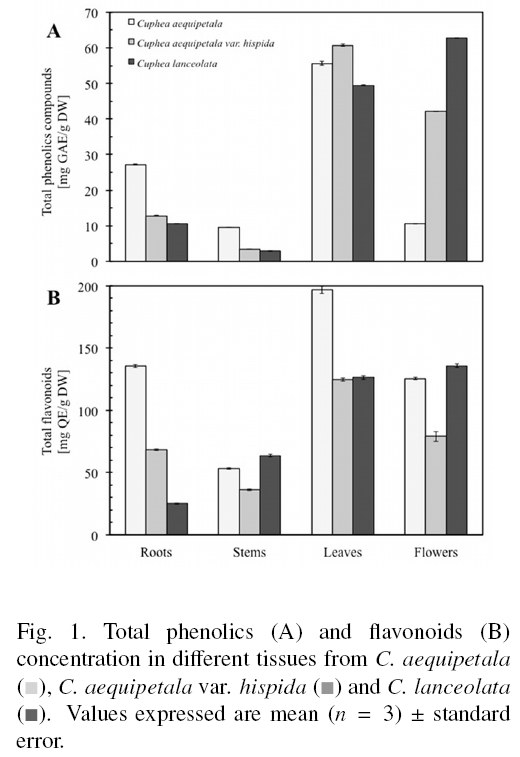
We found that the concentrations of compounds varied depending on the genotype (intra-varietal and intra-specific), and that they were distributed non-uniformly among plant organs. The leaves of C. aequipetala and C. aequipetala var. hispida and the flowers of C. lanceolata were the best source of total phenolic compounds (Fig. 1A) and flavonoids (Fig. 1B). Several reports have shown differences in the concentrations of phytochemicals among varieties and species within a genus, which may also be influenced (among other factors) by different growing conditions (e.g., soil, altitude, temperature, nutrition) (Gesch et al., 2010; Kim et al., 2011; Zheljazkov et al., 2011) or harvest time (He et al., 2010). For instance, total phenolic compounds and flavonoid content was shown to vary significantly in the leaves of six species of Artemisia (Asteraceae) (Carvalho et al., 2011), whereas the concentration of the bioactive compounds in Salvia miltiorrhizae (Labiatae) was affected mainly by genetic factors (He et al., 2010). The differences in phenolic compound and flavonoid concentration among Cuphea species may be the result of adaptation processes of plants to environmental conditions. Several authors have explained that light increases flavonoid concentrations in certain organs/tissues of the plant (Wang et al., 2009; Ghasemzadeh et al., 2010; Martz et al., 2010). Plants growing at higher altitudes are exposed to higher light intensities, so they must develop mechanisms to prevent damage caused by photo-destruction. C. lanceolata grows at lower elevations, agreeing with the notion of a lesser need of protection against light, whereas C. aequipetala and C. aequipetala var. hispida grow at higher altitudes where light intensities are more important. However, the results of present work demonstrate that flowers don't follow this behavior, showing that the genetic fixed information play an important role in the determining of secondary metabolites amounts/profiles.
The highest concentration of total phenolic compounds and flavonoids found in the present study for Cuphea spp. (62.79 mg GAE/g DW and 196.83 mg QE/g DW) were higher than those reported for other medicinal plants considered as high in antioxidant compounds such as Sargentodoxa cuneata Redh Et Wils (Sargentodoxaceae) (52.35 mg GAE/g DW) or Fraxinus rhyncophylla Hance (Oleaceae) (40.27 GAE/g DW) (Li et al., 2008) but lower than the contents reported for other Lythraceae used for medicinal purposes. Methanol extracts from the bark of Lafoensi pacari (Lythraceae) contained 141 mg GAE/g dry matter (Solon et al., 2000), whereas those from the whole plants of Lawsonia innermis Linn. (Lythraceae) contained 238-310 mg GAE/g dry matter (Guha et al., 2011).
3.3 Antioxidant properties
The antioxidant activity of organs of C. aequipetala and C. aequipetala var. hispida using the DPPH assay varied from 19.19+0.1 to 169.33+2.1 and 18.48+0.13 to 173.33+2.1 μmol trolox/g DW, respectively (Fig. 2A). For both varieties, antioxidant activity using DPPH could be ranked in descending order: leaves > flowers > roots > stems. Cuphea lanceolata exhibited mean DPPH activities between 5.31+0.1 and 159.50+0.6 μmol trolox/g DW, and this could be ranked in organs in descending order as flowers > leaves > roots > stems (Fig. 2A). Meanwhile, scavenging activity evaluated by ABTS of C. aequipetala and C. aequipetala var. hispida extracts was between 106.71+0.3 to 541.10+2.32 and 14.42+0.2 to 336.23+0.8 μmol trolox/g DW, respectively; with the leaves and flowers being the most active (Fig. 2B). Mean ABTS values for C. lanceolata ranged between 20.07+0.1 and 275.60+3.9 μmol trolox/g DW, with the flowers being the most active. According to statistical analyses, C. aequipetala exhibited higher free radical-scavenging activity (P < 0.05) than C. aequipetala var. hispida and C. lanceolata, and the activities were dependent on the plant organ (P < 0.05). The highest values of free-radical scavenging against ABTS found for Cuphea spp. in the present study are higher than those reported for other medicinal plants; for instance, Sargentodoxa cuneata Rehd. Et Wils (265.43+4.62 μmol trolox/g DW), Fraxinus rhynchophylla Hance (166.09+0.34 μmol trolox/g DW) or Paeonia suffruticosa Andr (221.10+0.34 μmol trolox/g DW) (Li et al., 2008).

The reducing power of C. aequipetala and C. aequipetala var. hispida was, on average, between 93.11+0.2 to 1186.25+3.2 and 107.83+0.8 to 341.52+1.2 μmol trolox/g DW, respectively (Fig. 3). For both varieties, reducing power in organs could be ranked in the descending order: leaves > flowers > roots > stems. Within C. lanceolata, the flowers and leaves were more active (553.06+0.6 and 195.03±6.3 μmol trolox/g DW, respectively) than roots and stems (101.43±0.8 and 51.23±0.3 μmol trolox/g DW, respectively). The species (P < 0.05), variety (P < 0.05) and the organ (P < 0.05) were found to significantly influence the reducing power of the extracts. Reducing power presented by the leaves of Cuphea aequipetala is close to that reported for bark extracts of Populus tremuloides Michx (1406.74 umol trolox/g DW) but higher than the reported for the synthetic antioxidant tert-butyl-4-hydroxy-toulene (BHT) (686.79 μmol trolox/g) which is used in food processing (Diouf et al., 2009).

The extracts of Cuphea spp. presented free-radical scavenging activity and reducing power. For most all samples, PPM values were higher than ABTS•+ and DPPH•+ values. These differences in the capacity of the extracts to scavenge ABTS•+/DPPH•+ radicals and to reduce PPM are in accordance with previous observations (Marwah etal., 2007; Pasko etal., 2009).
In these assays, the transfer of hydrogen and electrons occurs at different redox potentials and is also dependent upon the structure of the antioxidant (Marwah et al., 2007). Factors such as the presence of pigments (e.g., anthocyanins, carotenoids) (Dykes et al., 2005), the solubility of the extract in the testing systems or the solvent in which the reaction takes place, have been reported to affect the capacity of the extracts to react with different radicals (Cai et al., 2004; Adedapo et al., 2008). The ability of extracts of Cuphea spp. to scavenge different free radicals in different systems may be an advantage for therapeutic agents to treat radical-related diseases (Adedapo et al., 2008; Sucontphunt et al., 2011). Furthermore, the antioxidant properties of Cuphea spp. may be associated with its traditional use to treat conditions consistent with radical-related diseases (e.g., tumors).
3.4 Qualitative chemical analysis by TLC
Qualitative analysis by TLC using DPPH was performed to detect the chemical components of Cuphea responsible of the free-radical scavenging activity and as an initial study of their chemical constitution. This method enables rapid detection and localization of active compounds in a complex extract (Gu et al., 2008). For this, the extracts from leaves of the three species of Cuphea were analyzed. In all cases, the extracts resolved at least one band with antiradical activity (RF = 0.60), which was also observed under UV light at 264 and 365 nm and gave a yellow fluorescence after reaction with diphenylboric acid-S-ethylamino ester (data not shown).
This band agrees with that of the flavonoid quercetin 3-β-D-glucoside. TLC profile of the leaves of the three species was similar in revealing fluorescent zones at 365 nm that reacted with diphenylboric acid-β-ethylamino ester, giving yellow fluorescence suggesting the presence of other flavonoids (Wagner and Bladt 1996). The leaves of C. aequipetala presented additional bands corresponding to flavonoids of less polarity than C. aequipetala var. hispida and C. lanceolata. In the aerial parts of Cuphea carthagenensis the main flavonoids are quercetin-5-O-β-glucopyranoside, quercetin-3-O-α-arabinofuranoside and quercetin-3-sulfate, which have been suggested as chemical markers of this species (Krespsky et al., 2012). These results are under further investigation.
3.5 Correlation analyses
We undertook correlation and regression analyses to determine the relationship between the phytochemical content and antioxidant capacity of the extracts. Total phenol content was significantly correlated (P < 0.05) with radical-scavenging activity based on the reduction of DPPH*+ (r=0.794) and ABTS*+ (r=0.468) as well as with reducing activity (r = 0.553). Similarly, flavonoid concentration was significantly correlated (P < 0.05) with scavenging activity against DPPH•+ (r=0.742), ABTS•+ (r=0.753) and reducing power (r=0.733) (Table 2). Phenolic compounds (e.g., phenolic acids, flavonoids, tannins) are considered to be the major contributors to the antioxidant activity of plants. The antioxidant capacity of phenolic compounds is attributed to their redox properties, which allow them to act as reducing agents, hydrogen donators, singlet-oxygen quenchers and metal-chelators (Rice-Evans and Miller 1996; Vermerris and Nicholson 2008). We found that the antioxidant activity of Cuphea spp. (free radical-scavenging and reducing power) was strongly correlated with total phenolic and flavonoid content (Table 2). However, the highest correlation coefficients were for flavonoids, suggesting that these types of compounds are the major contributors to the antioxidant properties of Cuphea spp. In related species, such as Lawsonia inermis Linn. (Lythraceae), antioxidant activity was also found to correlate strongly with total polyphenol content (Guha et al., 2011).

Conclusions
Cuphea spp. could be used as sources of natural antioxidants, such as phenolic compounds. Total phenolic compound and flavonoid concentrations were dependent upon the species, varieties and organs of plants.
Acknowledgements
This work was supported by the Secretaría de Investigación y Posgrado del Instituto Politecnico Nacional (IPN-Mexico, grant number SIP20120745) and by Fondo Mixto de Fomento a la Investigación Científica y Tecnologica CONACYT-Gobierno del Estado de Morelos (M0R-2007-C01-79409). ARLL, KBT, BPMM and GTT are grateful to SIBE and EDI (IPN).
References
Adedapo, A.A., Jimoh, F.O., Koduru, S., Masika, PJ. and Afolayan, A.J. (2008). Evaluation of the medicinal potentials of the methanol extracts of the leaves and stems of Halleria lucida. Bioresource Technology 99,4158-4163. [ Links ]
Aguilar-Rodríguez, S, Echeveste-Ramírez, N.L., Lopez-Villafranco, M.E., Aguilar-Contreras, A., Vega-Avila, E. and Reyes-Chilpa. R. (2012). Etnobotaínica, micrografía analítica de hojas y tallos y fitoquimica de Cuphea aequipetala Cav. (Lythraceae): una contribution a la Farmacopea Herbolaria de los Estados Unidos Mexicanos (FHEUM). Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 11, 316-330. [ Links ]
Ayan, A.K., Yanar, O., Cirak, C. and Bilgener, M. (2007). Morphogenetic and diurnal variation of total phenols in some Hypericum species from Turkey during their phenological cycles. Bangladesh Journal of Botany 36, 39-46. [ Links ]
Barbosa, E., Calzada, F. and Campos, R. (2007). In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. Journal of Ethnopharmacology 109, 552-554. [ Links ]
Bhatt, I.D., Dauthal, P., Rawat, S., Gaira, K.S., Jugran, A., Rawal, R.S. and Dharb U. (2012). Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Scientia Horticulturae 136, 61-68. [ Links ]
Biavatti, M.W., Farias, C., Curtius, F., Brasil, L.M., Hort, S., Schuster, L., Leite, S.N. and Prado, S.R.T. (2004). Preliminary studies on Campomanesia xanthocarpa (Berg.) and Cuphea carthagenensis (Jacq.) J.F. Macbr. aqueous extract: weight control and biochemical parameters. Journal of Ethnopharmacology 93, 385-389. [ Links ]
Bolling, B.W., Blumberg, J.B. and Chen, C.Y.O. (2009). Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chemistry 116, 351-355. [ Links ]
Buer, C.S., Imin, N. and Djordjevic, M.A. (2010). Flavonoids: New Roles for Old Molecules. Journal of Integrative Plant Biology 52,98-111. [ Links ]
Braga, F., Wagner, H., Lombardi, J.A. and Braga de Oliveira, A. (2000). Screening the Brazilian flora for antihypertensive plant species for in vitro angiotensin-I-converting enzyme inhibiting activity. Phytomedicine 7, 245-250. [ Links ]
Cai, Y., Luo, Q., Sun, M. and Corke, H. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Science 74, 2157-2184. [ Links ]
Calzada, F. (2005). Additional antiprotozoal constituents from Cuphea pinetorum, a plant used in Mayan traditional medicine to treat diarrhoea. Phytotherapy Research 19, 725-727. [ Links ]
Carvalho, I.S., Cavaco, T. and Brodelius, M. (2011). Phenolic composition and antioxidant capacity of six Artemisia species. Industrial Crops and Products 33, 382-388. [ Links ]
Castillo-Juaírez, I., Gonzaílez, V., Jaime-Aguilar, H., Martínez, G., Linares, E.R. and Romero, I. (2009). Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. Journal of Ethnoparmacology 122, 402-405. [ Links ]
Diouf, P.N., Stevanovic, T. and Cloutier, A. (2009). Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Science and Technology 43, 457-470. [ Links ]
Dykes L., Rooney L.W., Waniska R.D. and Rooney W.L. (2005). Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. Journal of Agricultural and Food Chemistry 53, 6813-6818. [ Links ]
Estrada-Zúñiga, M. E., Arano-Varela, H., Buendía-González, L. and Orozco-Villafuerte, J. (2012). Fatty acids, phenols content, and antioxidant activity in Ibervillea sonorae callus cultures. Revista Mexicana de Ingeniería Química 11, 89-96. [ Links ]
Garcia-Márquez, E., Roman-Guerrero, A., Pérez-Alonso, C., Cruz-Sosa, F., Jiménez-Alvarado, R. and Vernon-Carter, E.J. (2012). Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of Muitle extracts and their decay upon storage at different pH. Revista Mexicana de Ingeniería Química 11, 1-10. [ Links ]
Gesch, R.W., Kim, K.-I. and Forcella, F. (2010). Influence of seeding rate and row spacing on Cuphea seed yield in the Northern Corn Belt. Industrial Crops and Products 32, 692-695. [ Links ]
Ghasemzadeh, A., Jaafar, H. Z. E., Rahmat, A., Wahab, P.E.M. and Abd Halim, M.R.A. (2010). Effect of different light intensities on total phenolics and flavonoids synthesis and antioxidant activities in young ginger varieties (Zingiber officinale Roscoe). International Journal Molecular Science 11, 3885-3897. [ Links ]
Graham, S.A. (1991). Lythraceae. Flora de Veracruz. Instituto de Ecología, A. C. Mexico. 45 p. [ Links ]
Graham, S.A. and Kleiman, R. (1992). Composition of seed oils in some Latin American Cuphea (Lythraceae). Industrial Crops and Products 1, 31-34. [ Links ]
Gu, L., Wu, T. and Wang, Z. (2008). TLC bioautography guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT-Food Science and Technology 42, 131-136. [ Links ]
Guha, G., Rajkumar, V., Kumar, R.A. and Mathew, L. (2011). Antioxidant Activity of Lawsonia inermis Extracts Inhibits Chromium (VI)-Induced Cellular and DNA Toxicity. eCAM. Article ID 576456, 9 pages, doi:10.1093/ecam/nep205. [ Links ]
He, C.-E., Wei, J., Jin, Y. and Chen, S. (2010). Bioactive components of the roots of Salvia miltiorrhizae: Changes related to harvest time and germplasm line. Industrial Crops and Products 32, 313-317. [ Links ]
Hutzler, P., Fischbach, R., Heller, W., Jungblut, T.B., Reuber, S., Schmitz, R., Veit, M., Weissenbock G. and Schnitzler, J.P. (1998). Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. Journal of Experimental Botany 49, 953-965. [ Links ]
Jiménez, M., Castillo, I., Azuara, E. and Beristain, C.I. (2011). Antioxidant and antimicrobial activity of capulin (Prunus serotina subsp capuli) extracts. Revista Mexicana de Ingeniería Química 10, 29-37. [ Links ]
Juntachote, T. and Berghofer E. (2005). Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chemistry 92, 193-202. [ Links ]
Kefeli, V.I., Kalevitch M.V. and Borsari B. (2003). Phenolic cycle in plants and environment. Journal of Cell and Molecular Biology 2,13-18. [ Links ]
Kim, K.-I., Gesch, R.W., Cermak, S.C., Phippen, W.B., Berti, M.T., Johnson, B.L. and Marek, L. (2011). Cuphea growth, yield, and oil characteristics as influenced by climate and soil environments across the upper Midwest USA. Industrial Crops and Products 33, 99-107. [ Links ]
Klepacka, J., Gujska, E. and Michalak, J. (2011). Phenolic compounds as cultivar- and variety-distinguishing factors in some plant products. Plant Foods for Human Nutrition 66, 64-69. [ Links ]
Krepsky, P.B., Isidório, R.G., Dias de Souza J. F., Cortes, S.F. and Castro Braga, F. (2012). Chemical composition and vasodilatation induced by Cuphea carthagenensis preparations. Phytomedicine 19, 953-957. [ Links ]
Krishnaiah, D., Sarbatly R. and Nithyanandam, R. (2010). A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing 89, 217-233. [ Links ]
Lattanzio, V., Lattanzio, V.M.T. and Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry: Advances in Research. Editor: Filippo Imperato. 23. [ Links ]
Li, H.B., Wong, C.C., Cheng, K.W. and Chen, F. (2008). Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Science and Technology 41, 385-390. [ Links ]
Liu, X., Jia, J., Yang, L., Yang, F., Ge, H., Zhano, C., Zhang, L. and Zu, Y. (2012). Evaluation of antioxidant activitie of aqueous extracts and fractionation of different parts of Elsholtzia ciliata. Molecules 17, 5430-5441. [ Links ]
López-Laredo, A., Gómez-Aguirre, Y., Medina-Pérez, V., Salcedo-Morales, G., Sepúlveda-Jiménez, G. and Trejo-Tapia, G. (2012). Variation in antioxidant properties and phenolics concentration in different organs of wild growing and greenhouse cultivated Castilleja tenuiflora Benth. Acta Physiologiae Plantarum doi: 10.1007/s11738-012-1025-8. [ Links ]
Martz, F., Jaakola, L., Julkunen-Tiitto, R. and Stark, S. (2010). Phenolic composition and antioxidant capacity of bilberry (Vaccinium mytrillus) leaves in northern Europe following foliar development and along environmental gradients. Journal of Chemical Ecology 36, 1017-1028. [ Links ]
Marwah, R.G., Fatope, M.O., Mahrooqi, R.A., Varma, G.B., Abadi, H.A. and Al-Burtamani, S.K.S. (2007). Antioxidant capacity of some edible and wound healing plants in Oman. Food Chemistry 101, 465-470. [ Links ]
Millam, S., Mitchell, S.M., Moscheni, E. and Lyon, J.E. (1997). The establishment and regeneration of range of Cuphea germplasm in vitro. Plant Cell Tissue and Organ Culture 48, 143-146. [ Links ]
Naghiloo S., Movafeghi A., Delazar A., Nazemiyeh H., Asnaashari S. and Reza Dadpour M. (2012). Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of Astragalus compactus Lam. (Fabaceae). Bioimpacts 2, 105-109. [ Links ]
Pasko, P., Barton, H., Zagrodzki, P., Gorinstein, S., Folta, M. and Zachwieja, Z. (2009). Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chemistry 115, 994-998. [ Links ]
Pérez-Jiménez, J., Arranz, S., Tabernero, M., Diaz-Rubio, M.E., Serrano, J., Gofii, I. and Saura-Calixto, F. (2008). Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Research international 1, 274-285. [ Links ]
Phippen, W.B. (2010). Cuphea, In: Vollmann, J., Rajcan, I. (Eds.), Oil Crops. Springer New York, pp. 517-533. [ Links ]
Phippen W. B., Isbell T. A. and Phippen M. E. (2006). Total seed oil and fatty acid methyl ester contents of Cuphea accessions. industrial Crops and Products 24, 52-59 [ Links ]
Prieto, P., Pineda, M. and Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E. Analytical Biochemistry 269,49-56. [ Links ]
Re, R., Pelligrini, N., Proteggente, A., Pannal, A., Yang, M. and Rice, E.C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26, 1231-1237. [ Links ]
Rice-Evans C. A. and Miller N.J. (1996). Antioxidant activities of flavonoids as bioactive components of food. Biochemical Society Transactions 24, 790-795. [ Links ]
Sainchez-Moreno, C., Larrauri, J.A. and Saura-Calixto, F. (1998). A procedure to measure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture 76, 270276. [ Links ]
Santiago, L.J.M., Louro, R.P. and De Oliveira, D.E. (2000). Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their induction by copper sulphate. Annals of Botany 86, 1023-1032. [ Links ]
Saxena, A., Saxena, A.K., Singh, J. and Bhushan, S. (2010). Natural antioxidants synergistically enhance the anticancer potential of AP9-cd, a novel lignan composition from Cedrus deodara in human leukemia HL-60 cells. Chemico-Biological Interactions 188, 580-590. [ Links ]
Schuldt, E.Z., Farias, M.R., Ribeiro-do-Vallea, R.M. and Ckless, K. (2004). Comparative study of radical scavenger activities of crude extract and fractions from Cuphea carthagenensis leaves. Phytomedicine 11, 523-529. [ Links ]
Serrano-Maldonado, M. J., Guerrero-Legarreta, I., Perez-Olvera, C.D.P. and Soriano-Santos, J. (2011). Actividad antioxidante y efecto citotoxico de Cladocolea loniceroides (van Tieghem) Kuijt (Loranthaceae). Revista Mexicana de Ingeniería Química 10, 161-170. [ Links ]
Sucontphunt, A., De-Eknamkul, W., Nimmannit, U., Dan Dimitrijevich, S. and Gracy, R.W. (2011). Protection of HT22 neuronal cells against glutamate toxicity mediated by the antioxidant activity of Pueraria candollei var. mirifica extracts. Journal of Nature Medicines 65, 1-8. [ Links ]
Shohael, A.M., Chakrabarty, D., Ali M.B., Yu, K.W., Hahn, E.J., Lee, H.L. and Paek K.Y. (2006). Enhancement of eleutherosides production in embryogenic cultures of Eleutherucoccus sessiflorus in response to sucrose-induced osmotic stress. Process Biochemistry 41, 512518. [ Links ]
Solon, S., Lopes, L., Teixeira de Sousa, P. and Schmeda-Hirschmann, G. (2000). Free radical scavenging activity of Lafoensia pacari. Journal of Ethnopharmacology 72, 173-178. [ Links ]
Tisserat, B., O'Kuru, R.H., Cermak, S.C., Evangelista, R.L. and Doll, K.M. (2012). Potential uses for Cuphea oil processing by products and processed oils. Industrial Crops and Products 35, 111-120. [ Links ]
Tunalier, Z., Kosar, M., Küpeli, E., Calis, I. and Baser, K.H.C. (2007). Antioxidant, antiinflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. Journal of Ethnopharmacology 110, 539-547. [ Links ]
Turkmen, N., Sari, F. and Velioglu, Y.S. (2006). Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chemistry 99, 835-841. [ Links ]
Vega-Avila, E., Aguilar, R.T., Estrada, M.J., Ortega, M.L.V. and Ramos, R.R. (2004). Cytotoxic Activity of Cuphea aequipetala. Proceedings of the Western Pharmacology Society 47, 129-133. [ Links ]
Vega, E. (2005). Estudio de Cuphea aequipetala Cav. sobre la proliferación de células humanas transformadas. Tesis de Doctorado. Universidad Autónoma Metropolitana. Mexico, D.F., 133 p. [ Links ]
Vermerris W. and Nicholson R. (2008). Phenolic compounds and their effects on human health. In: Vermerris W, Nicholson R (eds) Phenolic Compound Biochemistry. Springer Science+Business Media BV, pp. 235-255. [ Links ]
Waizel, B.J. (2006). Las plantas en la historia de la medicina. In: Las plantas medicinales y las ciencias una visión multidisciplinaria. (eds.) Instituto Politécnico Nacional, pp. 587. [ Links ]
Waizel-Bucay, J., Martinez-Porcayo, G., Villarreal-Ortega, M.L., Alonso-Cortes, D. and Pliego-Castañeda, A. (2003). Estudio preliminar etnobotanico, fitoquimico de la actividad citotoxica y antimicrobiana de Cuphea aequipetala Cav. (Lythraceae). Polibotanica 14, 99-108. [ Links ]
Wang, C.-C., Chen, L.-G. and Yang, L.-L. (1999). Antitumor activity of four macrocyclic ellagitannins from Cuphea hyssopifolia. Cancer Letters 140, 195-200. [ Links ]
Wang, S.Y., Chen, C.T. and Wang, C.Y. (2009). The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chemistry 112, 676-684. [ Links ]
Wolf R.B., Graham S.A. and Kleiman, R. (1983). Fatty acid composition of Cuphea seed oils. JAOCS 60, 27-28. [ Links ]
Zhao, J., Dixon, R.A. (2010). The [']ins' and [']outs' of flavonoid transport. Trends in Plant Science 15, 72-80. [ Links ]
Zheljazkov, V.D., Cantrell, C.L. and Astatkie, T. (2011). Variation in podophyllotoxin concentration in leaves and rhizomes of American mayapple (Podophyllum peltatum L.). Industrial Crops and Products 33, 633-637. [ Links ]














