Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 no.3 Ciudad de México dic. 2012
lngeniería de alimentos
Monte Carlo simulation of orange juice pectinmethylesterase (pme) inactivation by combined processes of high hydrostatic pressure (hhp) and temperature
Aplicación del método de Monte Carlo para simular la inactivación de pectinmetilesterasa (pme) en jugo de naranja con procesos combinados de altas presiones hidrostáticas (aph) y temperatura
V. Serment-Moreno1, H. Mújica-Paz1, J.A. Torres2 and J. Welti-Chanes1*
1 Escuela de Biotecnología y Alimentos, Tecnológico de Monterrey, Av. Eugenio Garza Sada 2501 Sur Col. Tecnológico, 64849, Monterrey, Nuevo León, México. * Corresponding author. E-mail: jwelti@itesm.mx Tel. 52+ (81) 83-58-20-00 ext. 4821, Fax 52+ (81) 83-58-20-00 ext. 5830
2 Food Process Engineering Group, Department of Food Science & Technology, Oregon State University, 100 Wiegand Hall, Corvallis, OR 97331, USA
Received 18 of July, 2012.
Accepted 4 of September, 2012.
Abstract
The variability effect of kinetic data was investigated by simulating orange juice; pectinmethylesterase (PME) inactivation with combined processes of high hydrostatic pressure-temperature (100-500 MPa; 20-40°C), applying the Monte Carlo method. Parameters from an Eyring-Arrheniius model that predicts the kinetic inactivation constant (k) as a function of both pressure and temperature were found reported in literature and considered for the enalysis. The kinetic analysis was carried out with both Monte Carlo simulations and the traditional deterministic approach, which only considers mean values and does not take into account data variability. Simulations with the Monte Carlo method demonstrated that residual PME activity predicted with deterministic calculations greatly differed from those obtained through confidence intervals of simclated probabilistic distributions. Meen values overrated residual enzyme activity from 4% to ≈ 2, 800% when compared to the 95% confidence intervals generated with the Monte Carlo method. This divergence augmented as both applied pressure and temperature levels increased. Similar risk analysis projects can be further developed to establish the foundations for future food processing regulations of enzymatic control.
Keywords: process simulation, Monte Carlo, orange juice, high hydrostatic pressure (HHP), pectinmethylesterase (PME).
Resumen
Se estudió el efecto de la variabilidad de datos cinéticos simulando la inactivación de pectinmetilesterasa (PME) en jugo de naranja a diferentes combinaciones de altas presiones hidrostáticas (100-500 MPa) y temperatura (20-40°C), aplicando el método de Monte Carlo. Se consideraron los parámetros reportados en la literatura para el modelo de Eyring-Arrhenius, el cual predice la constante cinética de inactivación (k) de PME en función de la presión y temperatura. A través del uso del método de Monte Carlo se confirmó que para los efectos del presente trabajo, utilizar valores promedio de las variables involucradas puede conducir a resultados erróneos. Los valores de actividad enzimática residual calculados con el procedimiento determinístico sobrestimaron la reducción de la actividad residual desde 4% hasta ≈ 2,800 % en comparación con los intervalos de confianza generados con el método de Monte Carlo. Esta divergencia se acrecentó conforme se incrementaron los niveles de presión y temperatura aplicados. Este tipo de análisis ayudarían a establecer las bases para las nuevas regulaciones de procesamiento en el área de alimentos.
Palabras clave: simulación de procesos, Monte Carlo, jugo de naranja, altas presiones hidrostáticas (APH), pectinmetilesterasa (PME).
1 Introduction
1.1 The Monte Carlo simulation method
Industrial food process designs are based on deterministic calculations that do not take into account the system variability. Thus, processing parameters are arbitrarily readjusted in order to compensate prediction errors originated by process variations. As a result of these new processing conditions, the final product may be severely damaged due to over- or subprocessing (Torres etal., 2010; Chotyakul et al., 2011; Salgado et al., 2011). New food regulations strictly demand confidence intervals for pathogen inactivation levels (Salgado et al., 2011), thus there is an urgent need to reconsider the way industrial food processes are being designed.
An extensive set of experimental conditions may be necessary in order to give accurate estimations of confidence intervals and to analyze process variability. Nonetheless, the amount of invested time and economic resources limit the number of experiments that can be carried out. The Monte Carlo method allows to simulate multiple scenarios by generating probabilistic distributions of processing parameters, such as kinetic or raw material data, which can be obtained experimentally or from data reported in literature (Aranda and Salgado, 2008; Torres et al., 2010; Chotyakul et al., 2011; Salgado et al., 2011).
The microbial risk analysis (MRA) is used for foodborne illness prevention and stands as the most important application of the Monte Carlo method in the food industry. MRA involves a multidisciplinary analysis which involves selection and quantification of pathogens throughout the industrial food chain, food processing preservation technologies that are available to reduce the microorganism levels, and finally determine an acceptable risk level for consumers (Foegeding, 1997; Cassin et al., 1998a; Cassin et al., 1998b; Voysey and Brown, 2000; FDA and CFSAN, 2005; WHO, 2005; Delignette-Muller and Cornu, 2008; Teunis et al., 2008). Nevertheless there are no similar studies reported to control enzyme activity that can be detrimental for nutritional and/or sensorial aspects of food products, even though enzymes can be far more resistant to pasteurization treatments than microorganisms.
1.2 Orange juice pectinmethylesterase (PME)
Juice cloud can be defined as a complex mixture of several compounds that provide turbidity, color and aroma (Espachs-Barroso et al., 2005). Orange juice is highly susceptible to undesired enzymatic reactions and microbial growth, where cloud loss is the first notorious detrimental change of an unpasteurized orange juice (Pao and Fellers, 2003). Pectinmethylesterase (PME; EC 3.1.1.11) destabilizes orange juice by hydrolyzing pectin compounds present on the juice cloud. Pectic acids that result from hydrolysis can further interact with free Ca2+ and precipitate, which leads to juice clarification (de Assis et al., 2001; Casas-Forero and Caez-Ramirez, 2011). Orange PME can be found in the cell wall of peel, pulp and vesicles through electrostatic interactions, so the enzyme cannot be separated from the juice matrix (Espachs-Barroso et al., 2005; Simsek and Yemenicioglu, 2010).
Heat pasteurization is usually employed to inactivate orange juice PME, which presents a high heat resistance. (Versteeg et al., 1980; Cameron et al., 1998; Zhou et al., 2009). However, the application of high temperatures (≈ 80 - 90°C) needed to inactivate PME can severely affect nutritional compounds and sensorial characteristics (Polydera et al., 2005). High pressure processing (HPP) is an alternative non thermal pasteurization treatment that is able to achieve satisfactory PME inactivation levels. Industrial applications of HPP usually range from 100-700 MPa and 5-10 min. Moderate temperatures (45-65° C) can be applied in combination with high pressure to achieve higher microbial and/or enzymatic inactivation levels (Ludikhuyze et al., 2002; Balasubramaniam et al., 2008; Yaldagard et al., 2008; Bermudez-Aguirre and Barbosa-Canovas, 2011; Mújica-Paz et al., 2011; Domínguez-Fernández et al., 2012). PME is also highly resistant to HPP, and pressure levels above 500 MPa and temperatures in the range of 40-60°C are required, but nutritional and sensorial characteristics are best preserved when compared to orange juice pasteurized with a severe heat treatments (Goodner et al., 1998; Van den Broeck et al., 2000; Nienaber and Shellhammer, 2001; Ludikhuyze et al., 2002; Polydera et al., 2004; Polydera et al., 2005). In the present study, the dispersion of the Eyring-Arrhenius model parameters was simulated with the Monte Carlo method to evaluate the effect of variability on the prediction of orange juice PME inactivation with combined HPP-temperature processes.
2 Methodology
The Monte Carlo simulations were carried out with Microsoft Excel. Normal distributions of the enzymatic inactivation constant (k) were randomly generated and residual PME activity was estimated for each of the simulated k values.
2.1 Prediction of kinetic parameters of PME inactivation
2.1.1 Kinetic parameters for pectinmethylesterase (PME) inactivation predictions
Kinetic data describing simultaneous pressure and temperature effects on PME inactivation were taken from Katsarsos et al. (2010). Orange juice was subjected to pressure between 100-500 MPa, and temperatures ranging from 20-40°C. Residual PME activity was assumed to follow a first order kinetic and the rate inactivation constants (k) were adjusted according to the Eyring-Arrhenius model shown in Eq. (1).

The mean and standard values for each parameter of the Eyring-Arrhenius model (Table 1) were assumed to follow a normal distribution. Random vectors that contained 1,000 randomly distributed values were generated for each parameter, which consequently allowed to calculate one thousand different values of k(P, T).

Furthermore, the residual PME activity was predicted by substituting k(P, T) on the fractional first order kinetic model (Eq. 2).
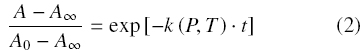
Residual PME distributions generated for each pressure and temperature combination was analyzed and the following data of the simulation distributions was reported: (a) mean value; (b) standard deviation; (c) upper confidence interval (95CI); (d) lower confidence interval (05CI); (e) maximum generated value; (f) minimum generated value. Confidence intervals were defined with 95% certainty; e.g. for 95CI, 950 out of 1,000 values of the residual enzymatic activity are equal to, or lower than the 95CI value.
2.1.2 Random number generation
The vectors for each parameter of the Eyring-Arrhenius model were generated in Microsoft Excel as follows: (1) A vector with one thousand random numbers from 0-1 was generated with the function RAND(), where the probability of withdrawal is the same for each element of the random array; (2) The previously generated random array of 01 also resembles the cumulative probability p(x) of a normal distribution. Thus, any element of the normal distribution (x) can be inferred by knowing its corresponding simulated cumulative probability p(x), and the statistical parameters (μ, σ) that describe the normal distribution function (Eq. 3).

3 Results and discussion
3.1 Evaluation of the Eyring-Arrhenius model
Predictions of k(P, T) with the parameters of the Eyring-Arrhenius model (Table 1) were verified to be in accordance with the experimental data reported by Katsaros et al. (2010). Experimental/predicted ratios (kexp/kpred) ranged between 0.5-2.0 for the whole experimental range (100-500 MPa; 20-40°C) except for both 100 and 300 MPa at 40°C. The analysis of residuals (kexp/kpred) showed no large deviations or nonlinear trends, and all values remained within -1 and 1. Even though both experimental/predicted ratios and residuals analysis indicate an accurate fit, the predictions of the Eyring-Arrhenius model were not uniform throughout the whole experimental conditions. The best model fit remained near the specified reference conditions (Table 1) at 200-300 MPa, whereas k(P, T) was overestimated 20-80% for the lowest pressure (100 MPa) and underestimated 2050% at 500 MPa.
3.2 Simulation of the orange juice PME inactivation constant with the Monte Carlo method
Probabilistic distributions of k(P, T) were obtained by generating arrays of each parameter of the Eyring-Arrhenius model (Table 1), with the Monte Carlo method. Experimental k(P, T) values were compared with the Monte Carlo generated mean, 05CI, 95CI and variation coefficient (VC) as shown in Fig. 1. Data dispersion increased as both pressure and temperature moved from the specified reference conditions (300 MPa, 30°C) established by Katsaros et al. (2010). As stated in the previous section, the Eyring-Arrhenius model predictions were not uniform through the whole pressure range and greater VC were seen for 100 MPa (Fig. 1a) and 500 MPa (Fig. 1d).
HPP variability could have been the main source of data dispersion, but the mathematical model used to describe the effect of pressure and temperature on k may not be the most adequate. The Eyring-Arrhenius model (Eq. 1) was originally developed for pure substances, whereas the constant R (8.30865 ml MPa mol-1 K-1 or 8.314 J mol-1 K-1) stands as an ideal gas thermodynamic property. The selection of the reference conditions may be another reason for the model behavior deviations at low and high pressures. Antagonistic effects on HPP enzyme inactivation have been commonly found, where PME activity has been reported to increase for high pressure treatments in the range of 100-300 MPa and temperatures higher than 50°C (Van den Broeck et al., 1999; Van den Broeck et al., 2000; Polydera et al., 2004; Eisenmenger and Reyes-De-Corcuera, 2009). Katsaros et al. (2010) did not observe antagonistic effects of increased PME activity from 100-500 MPa and 20-40°C. Additionally, Katsaros et al. (2010) obtained just three experimental data points for pressure levels above 300 and below 500 MPa. This scarce amount of data for the high pressure region may have been insufficient and could have influenced negatively on the parameter estimates of the Eyring-Arrhenius model (Eq. 1). Polydera et al. (2004) applied the same Eyring-Arrhenius model to describe PME inactivation for another orange variety, but the authors chose another set of reference conditions (600 MPa, 50°C) where the most significant PME inactivation took place and no antagonistic effects were present. Nonetheless, most of the experimental k(P, T) values remained within the confidence intervals (C/05, C/95) generated with the Monte Carlo method.
3.3 Prediction of orange juice PME residual activity with the Monte Carlo method
Residual PME activity was modeled at four pressure (100, 200, 300. 500 MPa) and three temperature (20, 30, 40°C) levels. Mean values predicted with the Monte Carlo simulation were compared with the traditional deterministic approach, which consisted in substituting each parameter of Table 1 in the Eyring-Arrhenius model (Eq. 1), and estimating the PME residual activity with the fractional first order equation (Eq. 2). All of the simulated mean values were in accordance with the deterministic method as shown in figs. 2, 3, 4.
The correlation between the Monte Carlo and deterministic values trends to improve as the amount of simulated data is increased. On the contrary an excessive quantity of simulated data will severely affect the simulation time, particularly if calculation steps are excessive or too complex. The minimum amount of data for a Monte Carlo simulation can be based on the variation coefficient (VC) of the generated data (Almonacid and Torres, 2010). Chotyakul et al. (2011) attained stable VC with 100 simulated data while predicting thermal sterilization times of canned mushrooms. On the other hand, Cassin et al. (1998a) developed 25,000 sized arrays for microbial risk assessment to estimate hemolytic uremic syndrome incidence after contaminated ground beef ingestion. The number of simulated data can widely vary, but most importantly the model validity should be addressed with several of the simulated scenarios (Nauta, 2002).
Since the simulated VC for the predicted PME residual activities were high, the Monte Carlo analysis was also performed with 10,000 and 100,000 sized distributions. Parameters of the simulations (mean, standard deviation, confidence intervals) did not have significant differences in spite of the augmented amount of generated data (Pvalue < 0.05). As a result, it was concluded that 1,000 sized distributions were enough to give a satisfactory simulation and that large VC were due to variability of the data reported by Katsaros et al. (2010).
An exponential decay tendency was observed for both the deterministic and the Monte Carlo predicted residual enzymatic activity. Mean values and the confidence intervals followed well-defined lines with little or no deviations, whereas the maximum (Max) and minimum (Min) reflected the data variability but still showed an exponential decrease trend. The residual enzymatic activity was lowered as both pressure and temperature levels increased while data dispersion was more intense. As a result, there was a significant difference between the predicted enzymatic activities calculated with both the experimental and simulated mean values (50% confidence; deterministic method), and the simulated CI95 (95% confidence; Monte Carlo method). Residual PME overestimation was more evident as both process pressure and temperature increased (4-38%, 20°C; 20-30%, 30°C; > 90%, 40°C). Since PME activity after HPP was calculated with the k(P, T) from the Eyring-Arrhenius model, the deviations of the C/95 from the predicted mean values could be due to the data variability and model considerations discussed in Section 3.2.
Conclusions
Simulations performed with the Monte Carlo method were able to reasonably predict the inactivation kinetic constant and the residual PME activity, particularly for treatments that are near the reference processing conditions (300 MPa, 30°C). Deterministic calculations design may lead to misinterpretation of the results, and therefore produce an inaccurate process design. Additionally, model validity must be the first and most important step before carrying on a Monte Carlo analysis. For the present work, predicted residual enzymatic activities differences between experimental mean values and simulated confidence intervals could have been originated by the natural data variability, the Eyring-Arrhenius model, the selected reference conditions, and the lack of experimental data between the 300-500 MPa pressure region. Monte Carlo simulation is as a reliable tool for simulating variability effect on food pasteurization processing, which can be widely recommended for food process design and validation.
Nomenclature

Acknowledgements
Authors Serment-Moreno, Mújica-Paz and Welti-Chanes acknowledge the financial support from Tecnologico de Monterrey (Research Chair Funds CAT-200), and CONACYT-SEP (Research Project 101700 and Scholarship Program).
References
Almonacid, S.F. and Torres, J.A. (2010). Uncertainity of microbial shelf-life estimations for refrigerated foods due to the experiemental variability of the model parameters. Journal of Food Process Engineering 33, 66-84. [ Links ]
Aranda, J.S. and Salgado, E. (2008). Enzymatic catalysis modelling with allosteric enzymes. Revista Mexicana de Ingeniería Química 7, 2127. [ Links ]
Balasubramaniam, V.M., Farkas D. and Turek E.J. (2008). Preserving foods through high-pressure processing. Food Technology 62, 32-38. [ Links ]
Bermudez-Aguirre, D. and Barbosa-Canovas, G. (2011). An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Engineering Reviews 3, 44-61. [ Links ]
Cameron, R.G., Baker R.A. and Grohmann K.J. (1998). Multiple forms of pectinmethylesterase from citrus peel and their effects on juice cloud stability. Journal of Food Science 63, 253-256. [ Links ]
Casas-Forero, N. and Cáez-Ramírez, G. (2011). Morfometric and quality changes by application of three calcium sources under mild thermal threatment in pre-cut fresh melon (Cucumis melo L.). Revista Mexicana de Ingeniería Química 10,431-444. [ Links ]
Cassin, M.H., Lammerding A.M., Todd E.C., Ross W. and McColl R.S. (1998a). Quantitative risk assessment for Escherichia coli O157:H7 in ground beef hamburgers. International Journal of Food Microbiology 41, 21-44. [ Links ]
Cassin, M.H., Paoli G.M. and Lammerding AM. (1998b). Simulation modeling for microbial risk assessment. Journal of Food Protection 61, 1560-1566. [ Links ]
Chotyakul, N., Velazquez G., Torres J.A. (2011). Assessment of the uncertainty in thermal food processing decisions based on microbial safety objectives. Journal of Food Engineering 102, 247-256. [ Links ]
de Assis, S.A., Lima D.C. and de Faria Oliveira O.M.M. (2001). Activity of pectinmethylesterase, pectin content and vitamin C in acerola fruit at various stages of fruit develoPMEnt. Food Chemistry 74, 133137. [ Links ]
Delignette-Muller, M.L. and Cornu, M. (2008). Quantitative risk assessment for Escherichia coli O157:H7 in frozen ground beef patties consumed by young children in french households. International Journal of Food Microbiology 128, 158-164. [ Links ]
Domínguez-Fernández R.N., Arzate-Vázquez I., Chanona-Pérez J.J., Welti-Chanes J.S., Alvarado-González J.S., Calderon-Domínguez G., Garibay-Febles V. and Gutiérrez-López G.F. (2012). Aloe vera gel: Structure, chemical composition, processing, biological activity and importance in pharmaceutical and food industry. Revista Mexicana de Ingeniería Química 11, 23-43. [ Links ]
Eisenmenger, M.J. and Reyes-De-Corcuera, J.I. (2009). High pressure enhancement of enzymes: A review. Enzyme and Microbial Technology 45, 331-347. [ Links ]
Espachs-Barroso, A., Soliva-Fortuny R.C. and Martín-Belloso O. (2005). A natural clouding agent from orange peels obtained using polygalacturonase and cellulase. Food Chemistry 92, 55-61. [ Links ]
FDA and CFSAN. (2005). Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters. 166. [ Links ]
Foegeding, P.M. (1997). Driving predictive modeling on a risk assessment path for enhanced food safety. International Journal of Food Microbiology 36, 87-95. [ Links ]
Goodner, J.K., Braddock R.J. and Parish M.E. (1998). Inactivation of pectinesterase in orange and grapefruit juices by high pressure. Journal of Agricultural and Food Chemistry 46, 1997-2000. [ Links ]
Katsaros, G.I., Tsevdou M., Panagiotou T. and Taoukis P.S. (2010). Kinetic study of high pressure microbial and enzyme inactivation and selection of pasteurisation conditions for Valencia orange juice. International Journal of Food Science & Technology 45, 1119-1129. [ Links ]
Ludikhuyze, L., Ludikhuyze L., Van den Broeck I., Weemaes C. (2002) Effects of high pressure on enzymes related to food quality. In: Ultra high pressure treatments of foods, (M.E.G. Hendrickx, D. Knorr, L. Ludikhuyzeet al. eds.), Pp. 115-166. Springer US. [ Links ]
Mújica-Paz, H., Valdez-Fragoso A., Tonello-Samson C., Welti-Chanes J. and Torres J.A. (2011). High-pressure processing technologies for the pasteurization and sterilization of foods. Food and Bioprocess Technology 4, 969-985. [ Links ]
Nauta, M.J. (2002). Modelling bacterial growth in quantitative microbiological risk assessment: Is it possible? International Journal of Food Microbiology 73, 297-304. [ Links ]
Nienaber, U. and Shellhammer, T.H. (2001). High-pressure processing of orange juice: Kinetics of pectinmethylesterase inactivation. Journal of Food Science 66, 328-331. [ Links ]
Pao, S. and Fellers, PJ. (2003) Citrus fruits. Oranges. In: Encyclopedia of food sciences and nutrition (second edition), (B. Caballero,ed.), Pp. 1341-1346. Academic Press, Oxford. [ Links ]
Polydera, A.C., Galanou E., Stoforos N.G. and Taoukis P.S. (2004). Inactivation kinetics of pectin methylesterase of greek navel orange juice as a function of high hydrostatic pressure and temperature process conditions. Journal of Food Engineering 62, 291-298. [ Links ]
Polydera, A.C., Stoforos N.G. and Taoukis P.S. (2005). Quality degradation kinetics of pasteurised and high pressure processed fresh navel orange juice: Nutritional parameters and shelf life. Innovative Food Science & Emerging Technologies 6, 1-9. [ Links ]
Salgado, D., Torres J.A., Welti-Chanes J. and Velazquez G. (2011). Effect of input data variability on estimations of the equivalent constant temperature time for microbial inactivation by HTST and retort thermal processing. Journal of Food Science. [ Links ]
Simsek, S. and Yemenicioglu, A. (2010). Commercially suitable pectin methylesterase from Valencia orange peels. Turkish Journal of Agriculture and Forestry 34, 109-119. [ Links ]
Teunis, P.F., Ogden I.D. and Strachan N.J.C. (2008). Hierarchical dose response of E. coli O157:H7 from human outbreaks incorporating heterogeneity in exposure. Epidemiology and Infection 136, 761-770. [ Links ]
Torres, J.A., Chotyakul N., Velazquez G., Saraiva J.A. and Perez-Lamela C. (2010) Integration of statistics and food process engineering: Assessing the uncertainty of thermal processing and shelf-life estimations. In: VI Congreso Español de Ingeniería de Alimentos. Logroño, La Rioja, España. [ Links ]
Van den Broeck, I., Ludikhuyze L.R., Van Loey A.M. and Hendrickx M.E. (2000). Inactivation of orange pectinesterase by combined high-pressure and temperature treatments: A kinetic study. Journal of Agricultural and Food Chemistry 48, 1960-1970. [ Links ]
Van den Broeck, I., Ludikhuyze L.R., Van Loey A.M., Weemaes C.A. and Hendrickx M.E. (1999). Thermal and combined pressure-temperature inactivation of orange pectinesterase: Influence of ph and additives. Journal of Agricultural and Food Chemistry 47, 2950-2958. [ Links ]
Versteeg, C., Rombouts F.M., Spaansen C.H. and PilnikW. (1980). Thermostability and orange juice cloud destabilizing properties of multiplle pectinesterases from orange. Journal of Food Science 45, 969-971. [ Links ]
Voysey, P.A. and Brown, M. (2000). Microbiological risk assessment: A new approach to food safety control. International Journal of Food Microbiology 58, 173-179. [ Links ]
WHO (2005) Risk assessment of Vibrio vulnificus in raw oysters. Interpretative summary and technical report. In: Microbiological Risk Assessment Series. World Health Organization, Food and Agriculture Organization of the United Nations, Rome, Italy. [ Links ]
Yaldagard, M., Mortazavi S.A. and Tabatabaie F. (2008). The principles of ultra high pressure technology and its application in food processing/preservation: A review of microbiological and quality aspects. African Journal of Biotechnology 7, 2739-2767. [ Links ]
Zhou, L., Zhang Y., Hu X., Liao X. and He J. (2009). Comparison of the inactivation kinetics of pectin methylesterases from carrot and peach by high-pressure carbon dioxide. Food Chemistry 115, 449-455. [ Links ]














