Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ingeniería química
Print version ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 n.1 Ciudad de México Apr. 2012
Biotecnología
Fatty acids, phenols content, and antioxidant activity in Ibervillea sonorae callus cultures
Contenido de ácidos grasos, fenoles, y actividad antioxidante en cultivos de callo de Ibervillea sonorae
M.E. Estrada-Zúñiga1, H. Arano-Varela2, L. Buendía-González3* and J. Orozco-Villafuerte1
1 Facultad de Química, Universidad Autónoma del Estado de México, Paseo Colón esq. Paseo Tollocan s/n, Col.Residencial Colón, C.P. 50120 Toluca, Estado de México, México.
2 Departamento de Biotecnología, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco No. 186, (Col. Vicentina, Iztapalapa, C.P. 09340, México, D.F. México.
3 Facultad de Ciencias, Universidad Autónoma del Estado de México, Campus El Cerrillo, Piedras Blancas Carretera Toluca-Ixtlahuaca km 15.5 C.P. 50200 Toluca, Estado de México, México. *Corresponding author. E-mail: lety_sax@yahoo.com.mx +52 (722) 296-55-56, Fax+52 (722) 296-55-54
Received 3 of October 2011
Accepted 30 of November 2011
Abstract
Ibervillea sonorae callus cultures were established in order to produce fatty acids (lauric, myristic, pentadecanoic, palmitic and stearic acids) and phenolic compounds. Highest callus induction (100%) was obtained in treatments containing 2.32 or 4.65 μM Kinetin (KIN) with 2.26 or 6.80 μM 2,4-Dichlorophenoxyacetic acid (2,4-D). Highest fatty acids (FA) production (48.57 mg g-1), highest total phenol content (TPC; 57.1 mg gallic acid equivalents [GAE] g-1) and highest antioxidant activity (EC50; 573.3-855.7 /μg mL-1) was obtained from calius derived from 4.65 μM KIN with 6.80 μM 2,4-D. A direct relationship was observed between the callus growth index (GI), FA content, TPC and EC50. FA content was significantly higher in callus (48.57 mg g-1) than the reported for tuber roots from wild plaint (0.25 mg g-1). TPC and EC50 were higher in leaves (3560.1 mg GAE g-1 and (5(5.0-75.5 /μg mL-1, respectively) than in callus.
Keywords: plant tissue culture, Ibervillea sonorae, fatty acids, phenols, antioxidant activity.
Resumen
Se establecieron cultivos de callo de explantes de hoja de Ibervillea sonorae para evaluar la producción de ácidos grasos (ácido láurico, mirístico, pentadecanoico, palmítico y esteárico) y compuestos fenólicos. La mayor inducción de callo (100%) se obtuvo en los tratamientos que contenían 2.32 o 4.65 μM de cinetina (KIN) en combination con 2.26 o 6.80 μM de ácido 2,4-diclorofenoxiacetico (2,4-D). El callo derivado del tratamiento 4.65 μM KIN con 6.80 μM 2,4-D mostró los valores mas altos en términos de producción de ácidos grasos (FA; 48.57 mg g-1), contenido de fenoles totales (TPC; 57.1 mg equivalentes de ácido gálico [GAE] g-1) y actividad antioxidante (EC50; 5773.3-855.7 /μg mL-1). Se determinó una relación directa entre el índice de crecimiento de callo (GI) y el contenido de los FA, TPC y EC50. El contenido de FA en el callo fue significativamente mayor (48.57 mg g-1) al reportado para las raíces del tubérculo de la planta silvestre (125 mg g-1), mientras que TPC y EC50 fueron mayores en las hojas (360.1 mg GAE g-1 y 66.0-75.5 μg mL-1, respectivamente) comparadas con el callo.
Palabras clave: cultivo de tejidos vegetales, Ibervillea sonorae, ácidos grasos, fenoles, actividad antioxidante.
1 Introduction
Mexican folk medicine based on plant species has been extensively used for treating a large array of diseases. Such is the case of Ibervillea sonorae Greene (Cucurbitaceae), a climbing and native plant of northwestern México, commonly known as "wareque", "wereke" or "guareque". The leaves are used for treating skin ailments, stomach ulcers, and the tuber roots for counteracting diabetes mellitus (Hernández-Galicia et al., 2007). Pharmacological studies showed that the roots had significant hypoglycaemic activity on temporarily healthy and hyperglycaemic rabbits, and alloxan-diabetic mice. The fraction that showed higher hypoglycemic activity was that composed by a mixture of monoglycerids and fatty acids (Hernández-Galicia et al., 2007). On the other hand, there are no reports documenting which compounds are responsible for the curative effects of leaves. Nevertheless, it is known that closely related Cucurbitaceae species possess phenol compounds (Kolayli et al., 2010; Duke et al., 2001), so that it is likely that I. sonorae also bear phenolic compounds that display biological activity. Phenols are secondary metabolites produced in many plants as defence mechanism against environmental stress conditions such as attack by pathogens, insects orherbivores, wounding, ultraviolet light or/and heavy metal exposure, among others. Furthermore, phenols possess a great reactivity and show a wide variety of biological effects, including antibacterial, anti-inflammatory, antiallergic, hepato-protective, antithrombotic, antiviral, anticarcigenic and antioxidant activities (Soobrattee et al., 2005; Vermerris and Nicholson, 2006). It is known that free radicals are formed during the normal metabolism and under disease incurrence, causing a state of oxidative stress, thus damaging cells which can be scavenged through antioxidant activity of phenols (Vermerris and Nicholson, 2006; Soobrattee et al., 2005; Cowan, 1999). The high demand of I. sonorae has led to seek plant sources and appropriate technologies that assure their supply in high yields and constant composition. Plant tissue culture (PTC or in vitro culture) represents a viable technique for producing components with biological activity from vegetal material (Georgiev et al., 2009). The aim of this work was to: (a) determine if I. sonorae leaves contained phenolic compunds; (b) to establish I. sonorae culture conditions leading to the production of fatty acids and phenolic compounds, and (c) to compare the antioxidant activity of the phenolic compounds obtained from leaves and from callus.
2 Materials and methods
2.1 Plant material
I. sonorae young plants were provided by the Universidad Autonoma Metropolitana-Iztapalapa Campus (UAM-I) (Hernández-Galicia et al., 2007). On June 2010, the plants were fertilized and conditioned for 2 months in greenhouse (UAM-I). Immature leaves were removed from conditioned plants, and a part of them was used for the solvent extraction procedure described below, while the other part was used as source of explants to induce callus. The latter were washed with a 2% (v/v) soap solution for 10 min, rinsed and immersed for 30 s into a 70% (v/v) ethanol solution, followed by rinsing with distilled water. Afterwards they were immersed into 1.2% (v/v) sodium hipoclorite solution added with Tween-20 (three drops per 100 ml of solution), under low shaking conditions during 10 min, followed by aseptic rinsing with sterile distilled water (four times). Explants (5x5 mm segments) were put into the callus induction media culture. Each treatment for inducing callus consisted of 10 glass containers, every one containing three explants.
2.2 Establishment and propagation of callus cultures
The basal medium culture (BMC), which was used for all the treatments, consisted of Murashige and Skoog medium culture (MS, 1962) at half strength added with 3% (w/v) sucrose, 100 mg L-1 citric acid and 150 mg L-1 acid ascorbic. Phytagel at 0.2% (w/v) was used for solidifying the culture medium. For evaluating the callus response of immature leaves, the induction medium culture consisted of adding different combinations of plant growth regulators (PGR) to BMC: the cytokinin kinetin (KIN), and the auxin 2,4-dichlorophenoxyacetic acid (2,4-D) to medium culture. A factorial design (3 x 4; coded as KxDy) was employed. For KIN the applied concentration levels were 0.00 (K0), 2.32 (K1) and 4.65 (K2) µM, and for 2,4-D was 0.00 (D0), 2.25 (D1), 6.75 (D2) and 11.32 (D3) uM. All prepared media were adjusted to pH of 5.8, followed by the addition of 0.2% (w/v) phytagel, and sterilized at 121°C for 18 min. After 20 d of incubation, the frequency of callus inducement was assessed from the explants developing callus regarding the total number oftreated explants. The treatments displaying the highest frequency of callus, which were easily disaggregated and showed visually major growth in terms of biomass production, were chosen for being propagated in the respective media formulation (K1D1, K1D2, K2D1 and K2D2). Every 30 d, the selected calli (3.0 g fresh weight [FW]) were subcultured into new fresh media and incubated, with 8 subculture cycles being completed. Incubation conditions consisted of a photoperiod of 16 h (light)/8 h (dark) under white fluorescent light (50 µmol m-2 s-1) at 26 ± 2°C, during 30 d. In the last two subcultures (cultured for 30 d), the biomass was harvested to separately use it to determine phenol and fatty acid contents, growth index and antioxidant activity. Due to no statistical differences were determined between all the results determined from those two subcultures, they are mentioned throughout the manuscript as an average. Once calli are obtained it is known that they can undergo somaclonal variation which can result in secondary metabolite variations among subculture cycles. So, a period of maintenance is recommended to follow in order to achieve stable cell lines, where stability can be inferred through obtaining results reproducible regarding assessed parameters from one subculture cycle to another (Bourgaud et al., 2001).
2.3 Extraction of phenols and fatty acids
Calli (30-d-old) fresh biomass samples (2 g) were lyophilized and weighted, and the growth index (GI) determined as reported by Nezbedova et al. (1999). Dried biomass (100 mg dry weight [DW]) was put with boiling solvent (50 mL) for 1 h, filtered and concentrated (10 mL). Methanol (MeOH) was used for extracting the phenolic compounds, while hexane was for extracting the fatty acids. Leaves (from wild plants acclimatized to greenhouse) were subjected to the same treatment than the calli for phenolic compounds extraction. All measurements were done in triplicate.
2.4 Derivatization and quantification of fatty acids
Hexane concentrated extracts were derivatized for producing fatty acid methyl esters (FAMEs) according to the procedure of Shehata et al. (1979). Briefly, each concentrated sample (0.5 mL) was esterified with boiling H2SO4-MeOH (1:2; 3mL) for 3 h then it was cooled and transferred to a separation funnel. Each sample was rinsed three times with distilled water (1.5 mL), and finally hexane (2 mL) was added. The organic phase was collected and dried with anhydride sodium sulphate. Fatty acids standards (Lauric acid, C12; Myristic acid, C14; Pentadecanoic acid, C15; Palmitic acid, C16; and Stearic acid, C18; Sigma-Aldrich Co., USA) were treated in the same way for developing their respective methyl esters. Stock standard FAME solutions (1.0 mg mL-1 for C12, and 10 mg mL-1 for the other FAMEs) were prepared. Further dilutions of each stock solution (2.5, 5.0, 7.5 and 10 mg mL-1) were made in order to obtain the calibration curve of every FAME (coefficient of determination [r2] = 0.9892, 0.9896, 0.9902, 0.9923, 0.9964 and 0.9977 for methyl esters of C15, C14, C18, C16 and C12, respectively). The quantitative analysis for FAMEs was carried out in an Autosystem XL gases chromatography with flame ionization detector (GC-FID) system (Perkin Elmer, USA), equipped with a capillary column (HP-5 bonded and cross-linked 5% siloxane, 30 m x 0.32 mm I.D., 0.025 um film thickness; Agilent Technologies, USA). The temperature of the injection port was set at 300 °C, and for the detector was set at 300°C. While the oven temperature was programmed to initiate at 180 °C for 2 min, then the temperature was raised to 300 °C (at a rate of 5 °C min-1) and held for 15 min. Carrier gas (Helium) flow rate was set at 1.0 ml min-1, the injection volume was 1 uL in the split mode. Standards and samples were run in the same way. TotalChrom chromatography software (Perkin Elmer, USA) was used to acquire data from the detector. Each sample was injected almost three times (n > 3). For detecting, identifying and quantifying each FAME in samples, peak areas at the corresponding standard retention time were used from the calibration curve.
2.5 Determination of total phenol contents and ABTS•+ and DPPH•+ radical quenching
The calli that showed higher FAMEs content (K1D2, K2D2) were chosen for carrying out the total phenol and antioxidant measurements. Total phenol content (TPC) was determined from MeOH extracts by applying the Folin-Ciocalteau method (Aslan et al., 2007) and gallic acid (GA) was used to elaborate a calibration curve (1-25 mg mL-1; r2 = 0.9972); the results were expressed as milligrams of gallic acid equivalents per gram of dry extract (mg GAE g-1). On the other hand, MeOH extracts were used to assess radical scavenging, so 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; Sigma-Aldrich Co., USA) and 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich Co., USA) radicals were tested. The ABTS•+ assay was made according Re et al. (1999) with brief modifications. The ABTS•+ radical cation was produced through the reaction of 7 mM ABTS with 2.45 mM potassium persulfate for 16 h under darkness and room temperature. The resulting solution was diluted with MeOH to achieve a value of absorbance of 0.7 ± 0.05 at wavelength of 734 nm. While DPPH•+ assay was conducted according to Sanchez-Moreno et al. (1998), with some modifications. A 0.1 mM DPPH•+ solution was prepared with MeOH. For both assays a volume of 3000 uL of radical solution (diluted ABTS•+ or DPHH•+) was mixed with 500 uL of sample (extracts from callus and leaves), and after 15 min under darkness and room temperature incubation the absorbance was recorded at 734 nm for ABTS•+ assay and 515 nm for DPPH•+ assay. Since EC50 is defined as the concentration of extract needed to decrease at 50 % the initial radical, different concentrations of extracts from calli or leaves were tested for each assay (0-3 mg of extract mL-1). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; Sigma-Aldrich Co., USA) was used as standard, so different solutions were prepared to build a calibration curve (20-150 μM, r2 = 0.9903). The results of radical quenching to achieve the EC50 were expressed as microgram of dry extract per ml of extract (μg mL-1). Both assays were made three times for all samples (n = 3).
2.6 Statistics
All the results were statistically analyzed with NCSS software by performing One-way analysis of variance and the multiple comparison test of Tukey-Kramer. The significance level for all the statistical tests was established at 5 %. All the experiments were done by triplicate.
3 Results and discussion
3.1 Callus induction and calli development lines
The callus induction from explants of I. sonorae depended of the PGR treatments used. Callus response did not occur in explants grown under the control treatment (without PGR, K0D0) and those treatments consisting only of KIN in different concentrations (K1D0, K2D0). All the treatments made with 2,4-D alone or combined with KIN produced callus induction after seven days of incubation. Most of the induced calli were friable and white-yellowish in color after 7 days of incubation changing to a brown-yellowish color and slightly compact appearance after 20 days of incubation. The higher frequency response of callus induction (100%) was registered for treatments K1D1, K1D2, K2D1, K2D2, followed a significantly lower frequency response (75%) by treatments K0D1, K0D2, K0D3, K1D, andK2D3 (Fig. 1).
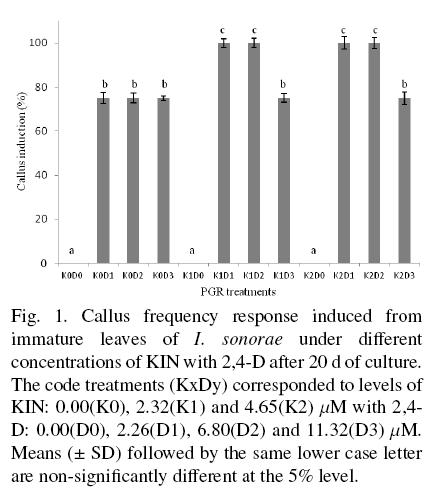
The callus induction in in vitro cultures is generally documented to be dependent on the addition of exogenous auxin and cytokinin, being critical the ratio between them to promote callus. An intermediate range of auxin and cytokinin is best for promoting calli formation, whilst a high ratio of auxin to cytokinin promotes root formation and a high ratio of cytokinin to auxin gives rise to shoots (Kakani et al., 2009). In plants, the auxin-cytokinin interaction determines the direction of cellular metabolism because their action regulates the expression of genes related to growth and development. Moreover, the action of both PGR is influenced by internal factors inherent to species and explants such as a differential sensitivity of receptor or transporter proteins and endogenous PGR levels (Mockaitis and Estelle, 2008; Sakakibara, 2006). Several PTC reports related to the Cucurbitaceae family, to which I. sonorae belongs, mainly deal with somatic embryogenesis (Elmeer and Hennerty, 2008).
However, few research works have been carried out with the aim of establishing PTC in cucurbit species showing medicinal properties, exceptions being those reported for Ecballium elaterium (Toker et al., 2003), Cucurbita andreana, and Cucurbita andreana/Cucurbita maxima hybrid (Halaweish and Tallamy, 1998). In the latter work best callus initiation was achieved with 9.0 μM 2,4-D and 4.65 μM KIN, result very similar to that found in this work with treatment K2D2.
3.2 FAMEs contents in cellular lines
All of the in vitro line cultures (K1D1, K1D2, K2D1 and K2D2) produced C12, C14, C15, C16 and C18 FAMEs. Significant total FAMEs differences were observed among treatments, being from highest to lowest: K2D2> K1D2> K1D1> K2D1. Treatment K2D2 showed a significant higher content of C12, C14, C15, C16, and C18, than the rest of the treatments (0.39, 12.51, 9.13, 15.92 and 10.62 mg g-1) (Table 1). Within K2D2, significant differences in the content of the individual fatty acids were observed, and were from higher to lower as follows:C16>C14>C18>C15>C12 (Table 1). Production of FAMEs seems to be closely related to GI. The higher GI was for a given treatment, the higher was its FAMEs contents (Table 1). All the cellular lines produced significantly higher FAMEs concentrations than that reported by Hernández-Galicia et al. (2007) for tuber roots (0.25 mg mg g-1). These authors also found that these FAMEs possessed hyploglycemic activity, so that it may be inferred that our cellular lines may also display hypoglucemic activity. Furthermore, the five fatty acids (C12, C14, C15, C16 and C18) found in our calli have been associated with hypoglycemic activity (Hernández-Galicia et al., 2007). Other cucurbits used to counteract diabetes have shown their effectiveness by means of displaying hypoglycemic activity, attributed to sterols, flavonoids, terpens and steroids (Andrade-Cetto and Heinrich, 2005). However, among all the reported natural products responsible for antidiabetic properties from many plants, just few of them are related to lipids and fatty acids (Qi et al., 2010; Negri 2005), such in the case of oleanolic acid from Ligustrum lucidum, hypothesized as stimulator of insulin release (Gao et al., 2007).
3.3 TPC and antioxidant activity in calli and leaves
Significant higher TPC and antioxidant activity were found in K2D2 callus (57.1 mg GAE g-1 and 573.3855.7 μg mL-1, respectively) than in K1D2 callus (21.2 mg GAE g-1 and 1973.1-2798.4 μg mL-1, respectively) (Table 2). As in the case of FAMEs production, TPC and antioxidant activity seem to be closely related to GI (Tables 1 and 2). Comparison of TPC and antioxidant activity between K2D2 callus and leaves show that the latter possessed significantly higher values for both parameters (360.1 mg GAE g-1 and 66.0-75.5 μg mL-1, respectively) (Table 2). Chew et al. (2011) categorized TPC into 4 classes based on mg GAE 100g-1 as follows: i) high for values > 5000, ii) medium high ranging between 3000-5000, iii) medium low ranging between 1000-3000, and iv) low for values < 1000. So, by this standard, the leaves showed medium high TPC, whereas both calli showed low TPC (Table 2). Likewise, Kuete and Efferth (2010) ranked the efficiency of radical scavenging at 50% of initial amount, where lower radical quenching expressed as μg mL-1 means a higher antioxidant activity, as follows: i) high for values < 50, ii) moderate ranging between 50-100, and iii) low for values> 100. Thus, leaves had a moderate radical scavenging capacity based on the values obtained for the DPPH•+ and ABTS•+ assays, while both calli possessed a low radical scavenging capacity (Table 2).
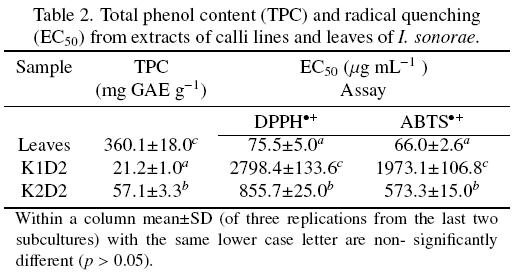
Several curcubit species have been reported as possessing phenolic compounds and antioxidant activity, but no reports exist regarding these two parameters for I. sonorae. The results found in this work indicate that TPC in leaves was significantly higher, and for K2D2 callus non-significantly different, than TPC (ranging from 49.7-157.6 mg GAE g-1) reported for several cucurbit species (Local Food-Nutraceuticals Consortium, 2005; Wu and Ng, 2008). On the other hand, the antioxidant activity found in leaves was significantly higher, but for both calli it was significantly lower, than values reported for some cucurbit species (129.94-500.00 μg mL-1, Local Food-Nutraceuticals Consortium, 2005; Wu and Ng, 2008). Nevertheless, the antioxidant activity found in the leaves of I. sonorae was significantly higher than that reported for vitamin E by Wu and Ng (IC50 = 172.21 μg mL-1, 2008). These results tend to explain the popular belief attached to I. sonorae leaves for remeding or counteracting diseases associated with oxidative stress. Likewise, fruits of capulin (Prunus serotina) which are widely used in Mexico to treat various diseases, showed significant antioxidant and antimicrobial activities and had high content of anthocyanin phenols (Jiménez et al., 2011). Molecular studies have revealed that phenolics can exert modulatory actions in cell by interacting with a wide spectrum of molecular targets central to the cell signaling machinery. These processes mark the point of no return in the cell death process, although the exact mechanism involved is not fully elucidated. Phenolic compounds may prevent oxidative stress and through their antioxidant activity exert protective effect by selectively inhibiting or stimulating key protein in the cell signaling cascades (Soobrattee et al., 2005).
Conclusion
This work establishes that I. sonorae leaves possess high amount of phenolic compounds and antioxidant activity that are higher or equivalent to those found in other cucurbit species. Also an explanation is provided as to why the leaves are used in folk medicine for treating diverse illnesses related to oxidative stress. Likewise, it was shown that it is possible to establish callus cellular lines for producing fatty acids and phenolic compounds with antioxidant activity. Fatty acids content in callus was higher than in the reported for tuber roots, but the opposite was found for the phenolic compounds content. Nevertheless, this work provides the stepping stone for enhancing the production of fatty acids and phenolic compounds from I. sonorae through the use of plant tissue culture techniques.
Acknowledgments
The first author would like to thank the Consejo Nacional de Ciencia y Tecnología (CONACyT) for her postdoctoral scholarship.
Nomenclature
EC50 concentration of extract needed to decrease
at 50% the initial radical, μg mL-1.
GI growth index.
TPC total phenol content per gram of dry extract, mg GAE g-1.
References
Andrade-Cetto, A. and Heinrich, M. (2005). Mexican plants with hypoglycaemic effect used in the treatment of diabetes. Journal of Ethnopharmacology 99, 325-348 [ Links ]
Aslan, M., Orhan, D.D., Orhan, N., Sezik, E. and Yesilada, E. (2007). In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp plicatum capitulurns in streptozotocin-induced diabetic rats. Journal of Ethnopharmacology 109, 54-59. [ Links ]
Bourgaud, F., Gravot, A., Milesi, S. and Gontier, E. (2001). Production of plant secondary metabolites: a historical perspective. Plant Science 161, 839-851. [ Links ]
Chew, Y.L., Ling Chan, E.W., Tan, P.L., Lim, Y.Y., Stanslas, J. and Goh, J.K. (2011). Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malasya. BMC Complementary and Alternative Medicine 11,12. [ Links ]
Cowan MM (1999) Plant products as antimicrobials agents. Clinical Microbiology Reviews 12, 564-582. [ Links ]
Duke, J.A., Bogenschutz-Godwin, M.J., duCellier, J. and Duke, P.K. (2001). Handbook of phytochemical constituents of GRAS herbs and other economic plants. 2nd edition, CRC Press, Florida. [ Links ]
Elmeer, K.M.S. and Hennerty, M.J. (2008). Observations on the combined effects of light, NAA and 2,4-D on somatic embryogenesis of cucumber (Cucumis sativus) hybrids. Plant Cell Tissue and Organ Culture 95, 381-384. [ Links ]
Gao, D., Li, Q., Li, Y., Liu, Z., Fan, Y., Han, Z., Li, J. and Li, K. (2007). Antidiabetic potential of oleanolic acid from Ligustrum lucidum Ait. Canadian Journal ofPhysiology and Pharmacology 85, 1076-1083. [ Links ]
Georgiev, M.I., Weber, J. and Maciuk, A. (2009). Bioprocessing of plant cell cultures for mass production of targeted compounds. Applied Microbiology and Biotechnology 83, 809-823. [ Links ]
Jiménez, M., Castillo, I., Azuara, E. and Beristain, C.I. (2011). Antioxidant and antimicrobial activity of capulin (Prunus serótina subsp capuli) extracts. Actividad antioxidante y antimicrobiana de extractos de capulin (Prunus serótina subsp capuli). Revista Mexicana de Ingeniería Química 10, 29-37. [ Links ]
Halaweish, F.T. and Tallamy, D.W. (1998). Production of cucurbitacins by cucurbit cell cultures. Plant Science 131, 209-218. [ Links ]
Hernández-Galicia, E., Calzada, F., Roman-Ramos, R. and Alarcon-Aguilar, F.J. (2007). Monoglycerids and fatty acids from Ibervillea sonorae root: isolation and hypoglycemic activity. Planta Medica 73, 236-240. [ Links ]
Kakani, A., Guosheng, L. and Peng, Z. (2009). Role of AUX1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxin and cytokikin interaction in Arabidopsis. Planta 229, 645-657. [ Links ]
Kolayli, S., Kara, M., Tezcan, F., Erim, F.B., Sahin, H., Ulusoy, E. and Aliyazicioglu, R. (2010). Comparative study of chemical and biochemical properties of different melon cultivars: standard, hybrid and grafted melons. Journal of Agricultural and Food Chemistry 58, 9764-9769. [ Links ]
Kuete, V. and Efferth, T. (2010). Cameroonian medicinal plants: pharmacology and derived natural products. Frontiers in Pharmacology 1(Art. 123). Doi: 10.3389/fphar.2010.00123. [ Links ]
Local Food-Nutraceuticals Consortium. (2005). Understanding local Mediterranean diets: a multidisciplinary pharmacological and ethnobotanical approach. Pharmacological Research 52, 353-366. [ Links ]
Mockaitis, K. and Estelle, M. (2008). Auxin receptors and plant development: a new signaling paradigm. Annual Review of Cell and Developmental Biology 24, 55-80. [ Links ]
Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiology Plantarum 15, 473-497. [ Links ]
Negri, G. (2005). Diabetes melito: plantas e principios ativos naturais hipoglicemiantes. Revista Brasileira de Ciências Framacêuticas 41, 121-142. [ Links ]
Nezbedova, L., Hesse, M., Dusek, J. and Werner, C. (1999). Chemical potential of Aphelandra sp. cell cultures. Plant Cell Tissue and Organ Culture 58, 133-140. [ Links ]
Qi, L., Liu, E., Che, C., Peng, Y., Cai, H. and Li, P. (2010). Anti-diabetic agents from natural products - An update from 2004 to 2009. Current Topics in Medicinal Chemistry 10, 434-457. [ Links ]
Re, R., Pelligrini, N., Proteggente, A., Pannala, A., Yang, M. and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26, 1231-1237. [ Links ]
Sánchez-Moreno, C., Larrauri, J.A. and Saura-Calixto, F. (1998). A procedure to measure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture 76, 270-276. [ Links ]
Sakakibara, H. (2006). Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology 57, 431-49. [ Links ]
Shehata, A.Y., De Man, J.M. and Alexander, J.C. (1979). A single and rapid method for the preparation of methyl esters of fats in milligram amounts for gas chromatography. Canadian Institute of Food Technology Journal 3, 85-89. [ Links ]
Soobrattee, M.A., Neergheen, V.S., Luximon-Ramma, A., Auroma, O.I. and Bahorum, T. (2005). Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis 579, 200-213. [ Links ]
Toker, G., Memisoglu, M., Toker, M.C. and Yesilada, E. (2003). Callus formation and cucurbitacin B accumulation in Ecballium elaterium callus cultures. Fitoterapia 74, 618-623. [ Links ]
Vermerris, W. and Nicholson, R. (2006). Phenolic compound biochemistry. Springer, Netherland. [ Links ]
Wu, S.J. and Ng, L.T. (2008). Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviate Ser.) in Taiwan. LWT-Food Science and Technology 41, 323-330. [ Links ]














