Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.3 Ciudad de México Dez. 2011
Biotecnología
Oxygen limitations to grow Azadirachta indica cell culture in shake flasks
Limitaciones por oxígeno para crecer un cultivo de células de Azadirachta indica en matraces
F. Orozco–Sanchez1,2, G. Sepúlveda–Jiménez2, G. Trejo–Tapia2, A. Zamilpa3 and M. Rodríguez–Monroy2*
1 Universidad Nacional de Colombia–Sede Medellín, Autopista Norte x Calle 65, Oficina 21–235, Medellín, Colombia.
2 Centro de Desarrollo de Productos Bióticos, Departamento de Biotecnología. Instituto Politécnico Nacional, México, Carretera Yautepec Jojutla Km. 6, (Calle CEPROBINo. 8, Colonia San Isidro, CP 62731, Yautepec, Morelos, México. *Corresponding author. E–mail: mrmonroy@ipn.mx Tel. 0052–735–394–20–20, Fax 00–00–00–00
3 Centro de Investigación Biomédica del Sur, Instituto Mexicano del Seguro Social, Argentina No. 1. Centro, CP 62790 Xochitepec, Morelos, México.
Received 6 of July 2011.
Accepted 28 of October 2011.
Abstract
It was evaluated the growth of Azadirachta indica cell suspension in different conditions of oxygen delivery in Erlenmeyer shake flask. Oxygen transfer rate (OTR, kg O2 m–3 day–1) for the closures utilized were: silicone foam (1.04), cotton (0.58), and aluminum foil (0.07). A. indica cells growing during 6 weeks of subculture showed that lower OTR reduced cell viability, the pH of broth medium, and Azadirachtins production. While, higher OTR induced the formation of aggregates. Using a stirred tank bioreactor, it was determined that A. indica cells had an oxygen consumption of 0.100 kg O2 kg DW–1 day–1, a higher value than other plant cell cultures. These results show that OTR generated in Erlenmeyer s hake flasks is lower to oxygen uptake rate of A. indica cells and it is a limiting factor to grow this plant.
Keywords: Azadirachta indica cell culture, shake flask, oxygen transfer rate, oxygen transfer resistance, closures.
Resumen
Se evaluó el crecimiento de suspensiones celulares de Azadirachta indica en diferentes condiciones de suministro de oxígeno en matraces. Las velocidades de transferencia de oxígeno (OTR, kg O2 m–3 día–1) obtenidas con los tapones utilizados para tapar matraces Erlenmeyer fueron: espuma de silicona (1.04), algodón (0.58) y papel aluminio (0.07). Las células de A. indica crecidas en las condiciones anteriores durante 6 semanas de subcultivo, mostraron que la OTR menor reduce la viabilidad celular, el pH del medio y la producción de azadiractinas. Por su lado, las OTR mayores favorecen la formación de agregados celulares. En un biorreactor de tanque agitado, se determinó que las células de Al. indica tienen un consumo de oxígeno de 0.100 kg O2 kg CS–1 día–1, valor superior al que tienen otros cultivos de células vegetales. Estos resultados muestran que la OTR generada en matraces Erlenmeyer es menor a la demanda de oxígeno de las células de A. indica y es un factor que limita su crecimiento.
Palabras clave: cultivo de células de Azadirachta indica, matraz agitado, velocidad de transferencia de oxígeno, resistencia a la transferencia de oxígeno, tapones.
1 Introduction
Microorganisms and plant cells are cultured in shake flasks with several biotechnological purposes such as culture medium optimization, elicitation or simply for the development of inocula for larger bioreactors. These aerobic systems demand oxygen to grow. However, low biomass yields and variation in metabolite production may occur as a result of an insufficient oxygen supply (Mantzouridou et al., 2005; Suresh et al., 2009). Several reports about oxygen transfer rate (OTR) and oxygen limitation in bacteria, yeast and fungus cultures have been published. These reports indicate that, in particular, variables such as the size and flask volume, agitation speed, and porous closures, affect OTR values (Veglio et al., 1998; Büchs, 2001; Nikakhtari and Hill, 2006; Suresh et al., 2009). Particularly, OTR determinations for plant cell culture in shake flask and the effects over cell growth and metabolite production have received little attention (Kieran et al., 1997).
Previous studies of Lee and Shuler (1991), reported that the type of closure affects plant cell metabolism and the headspace gas composition. Catharanthus roseus cells cultured in shake flasks plugged with Parafilm (American National Can, Greenwhich, Ct, USA) covered foam caps and Reynolds aluminum foil (Reynolds Metals Company, Richmond, Va, USA) accumulated ethylene and CO2, whereas oxygen limitations were detected. In this case, ajmalicine production was favored only by the use of aluminum foil. Despite that, there is no criterion for choosing the type of closure. Information about a possible effect of aluminum foil or other type of closure upon oxygen transfer limitations for other plant cell cultures is not available. Additionally, the relationship between OTR and oxygen uptake rate (OUR) of plant cells is not well known. In consequence, it is necessary continue studies that consider the effect of OTR in the establishment of cell cultures, their effects on growth and over the production of secondary metabolites.
It is reported that plant cell cultures such as Beta vulgaris (Rodríguez–Monroy and Galindo, 1999) and Solanum chrysotrichum (Rodríguez–Monroy et al., 2004) can be cultivated for a long time in shake flasks with aluminum foil cap. In contrast, an Azadirachta indica cell line incubated in shake flasks with aluminum foil cap, showed limitations to grow during its culture, and it was necessary periodically begin new suspension cultures from callus. This specie had interest because cell cultures producers of insecticide compounds classified as limonoids and Azadirachtins (Orozco–Sánchez and Rodríguez Monroy, 2007). In order to determine if the problems to grow A. indica in shake flask with aluminum foil cap was due to oxygen limitation, several OTR conditions were evaluated. These conditions resulted by using different types of closures (aluminum foil, cotton and silicone foam). This study reports the behavior of A. indica culture (growth index, pH medium, cell viability, aggregate morphology and secondary metabolites) during serial cultivation in shake flasks under different OTR conditions (0.07, 0.58 and 1.07 kg O2 m–3 day–1).
2 Materials and methods
2.1 Azadirachta indica culture
Cell suspension cultures of A. indica were obtained according to the methodology reported by Capataz–Tafur et al. (2007) using seeds for callus formation. Cells were grown in Murashige and Skoog (MS) medium (Murashige and Skoog, 1962), supplemented with sucrose (30 g/L), indole butyric acid (4 mg L–1) and benzylaminopurine (1 mg L–1). The medium pH was adjusted to 5.7 prior to sterilization. Cultures were grown in Pyrex 500 mL Erlenmeyer flasks (ref. 4980–500; Corning, New York, USA) with 100 mL of culture medium and subcultured every 7 days. Shake flasks were incubated in darkness, in an orbital shaker at 120 rpm with orbital diameter of 24 mm (model 6040, Vichi, México) at 25 ºC .
B. vulgaris was maintained according to the methodology reported previously by Rodríguez–Monroy and Galindo (1999).
2.2 Oxygen transfer in shake flasks
In order to estimate OTR and the relative relationship between the resistances to oxygen transfer through the plug and the gas–liquid phase, we used the following analysis:
Oxygen diffuses across the closure flask and from the gas phase to the liquid medium (Fig. 1). The oxygen transfer rate through the closure, Φclosure (kg O2 h–1), can be expressed by the following equation (Boon and Heijnen, 1998):
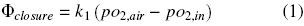
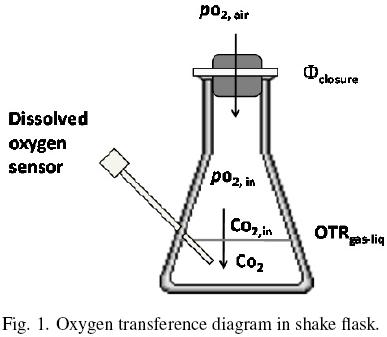
where pO2air (kg m –1 h –2) is the partial pressure of oxygen of the air, pO2,in (kg m –1 h –2) is the partial pressure of oxygen of the gaseous phase in the headspace of the flask and k1 is the oxygen transfer coefficient through the closure (m h). The oxygen transfer rate in the gas–liquid interface, OTRgas–liq, is given by:

Co2,in (kg m –3) is the oxygen concentration of the liquid phase at equilibrium with the oxygen in the headspace of the flask at the temperature system (po2,in), Co2 is the oxygen concentration of the liquid phase (kg m –3) and kLa is volumetric oxygen transfer coefficient in the gas–liquid interface (h–1). po2,in and Co2,in are related according to Henry's Law (Doran, 1995) as:
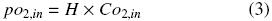
where H is Henry's constant (3.18 × 1013 m 3 h –2). Additionally, pO2,air can be expressed by:
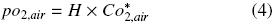
where Co*2air is the oxygen concentration of the liquid phase at equilibrium with air at pressure and the temperature of the system, 6.829 × 10–3 kg O2 m –3 (Doran, 1995; Büchs, 2001). If the same quantity of oxygen enters to gas phase inside the shake flask equals to oxygen enters to liquid phase, it means, the "buffer capacity" of the gas amount in the head space of the flask is neglected:
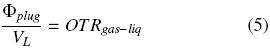
where VL is the liquid volume inside the flask (m 3). The before condition has been called "steady state" for the headspace in the flask (Boon and Heijnen, 1998; Veglio et al., 1998; Amoabediny and Büchs, 2007). Thus, using the previous equations, the following equation is obtained:
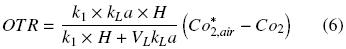
Defining Ko as follows

The following equation describes the OTR of a shake flask plugged:
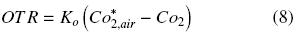
where Ko is the overall oxygen transfer coefficient (h–1). Similar criteria were previously reported by other authors, to estimate OTR in shake flasks (Boon and Heijnen, 1998; Veglio et al., 1998; Gupta and Rao, 2003). The maximum OTR (OTRmax) is given by equation (8) when CO2 is equal to zero. Therefore, Ko can be evaluated using the "dynamic gassing out" method and measuring CO2 in the liquid medium (Fig. 1) against time, according to equation (9) (Doran, 1995):

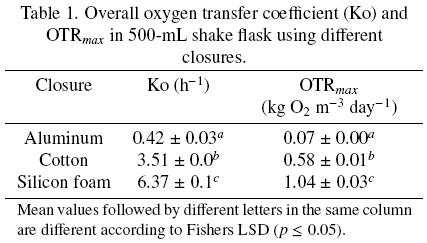
where Co2,ee is the steady–state oxygen concentration in the liquid medium, Co2,i is the oxygen concentration at the initial time (ti) and Co2 is the oxygen concentration at time (t). Shake flasks of 500 mL with 100 mL of culture medium were used for such measurements. MS basal medium supplemented with 30 g sucrose L –1 was used. Dissolved oxygen (DO) was determined using a polarographic electrode (Applisens dissolved oxygen sensor Z010032520, Applikon, Schiedam, Netherlands) connected to a recorder device (biocontroller ADI 1030, Applikon, Schiedam, Netherlands). Ko and OTRmax were determined using the closures previously mentioned: aluminum foil, cotton and silicone foam. Value of kLa was also determined without the closure under the agitation conditions before described.
The overall resistance to oxygen transfer, Rt (h), is the inverse of Ko and is equal to the resistance to oxygen transfer through the closure (R1), plus the resistance through the gas liquid interface (R2). They can be expressed using the following equations:
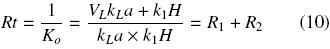


2.3 Effect of closure type on the growth and Azadirachtin production of A. indica cell culture
A. indica cell culture was grown in 125 mL shake flasks and three different closures: Reynolds aluminum foil (Costco Wholesale Corporation, Issaquah, WA, USA), cotton plugs made using 6.50 ± 0.03 g, and silicone foam (ref. C1046; Sigma, St. Louis, MO, USA). Previous experiments showed that that the cells conserved in shake flasks with cotton plugs and subcultured every 7 days, can conserve the growth and viability. So, for the three different closures, the measurements of cell growth, cell viability, pH medium, and Azadirachtin content were made every subculture of 7 days, during a 6–week period using three samples.
Biomass was determined by filtration of 10–mL samples through filter paper (grade 1; Whatman, Maidstone, UK). Cells were dried to constant weight at 70 °C, 1 day. Growth index (GI) was calculated with equation (15):

where Xt is biomass in t time (dry weight, DW, or kg DW m –3) and X0 is the initial biomass.
Cell viability was determined considering the membrane integrity by Evan's blue dye exclusion test (0.25% w/v) (Withers, 1985). Additionally, cell cultures was observed under a stereoscopic zoom microscope (SMZ1500; Nikon, New York, USA).
Azadirachtin evaluation was realized after of six subcultures. Procedure of extraction was done according to previously reported methodologies (Schaaf et al., 2000; Balaji et al., 2003; Capataz–Tafur et al., 2007) and the content in biomass and cell–free medium was determined. Biomass was separated by filtration through a filter paper (Whatman 1) and lyophilized. The lyophilized powder (0.5 g), was extracted with methanol (3 × 10 mL). Extracts were concentrated under low pressure distillation at 45 °C. The extract was fractionated by using 1.0 mL of sodium chloride (0.5% w/v), water (10 mL) and dichloromethane (3 × 10 mL). The organic phase was evaporated to dryness, and the dry powder dissolved in 2 mL of methanol for HPLC analysis.
A similar procedure was carried out for cell–free medium. Azadirachtins were determined by HPLC analysis, using a 2695 Waters Separation Module (Milford, MA, USA), 2996 Waters Photodiode Array Detector and Empower Pro Software. Separation was performed with a LiChroCART 125–4 LiChrospher 100 RP–18 column (125 mm X 4.6 mm, pore diameter 5 jum) (Merck, Whitehouse Station, NJ, USA). Mobile phase flow was 1.0 mL min–1 and a gradient was used beginning with 35:65 v/v acetonitrile/water (ACN/H2O), increased to 45:55 ACN/H2O (10 min), 70:30 ACN/H2O (11 min) and returned to 35:65 ACN/H2O (14 min). Fifty–microliter samples were injected to the HPLC and UV signal was recorded during a 15–min period (λ = 213 nm). Azadirachtin (A7430; Sigma) was used as standard.
2.4 OUR measurement of different plant cell cultures
In order to determine the oxygen demand by each plant cell species, a 7–L stirred tank bioreactor (Applikon) operated with a 45º inclined six–blade impeller, Di/Dt = 0.4 (400 rpm) and 0.08 vvm of aeration was used. Oxygen was measured using a polarographic electrode (Applisens dissolved oxygen sensor Z010032520, Applikon, Schiedam, Netherlands). Cell suspension cultures produced in shake flasks (500 mL) were used to inoculate the bioreactor with a working volume of 4 L, obtaining a suspension with 7 g DW/L. OUR was determined by the dynamic method (Doran, 1995). Determination of oxygen consumption was done during the first hour after cells were inoculated. Cultures were subjected to conditions without oxygen limitation (DO > 30%). OUR (kg O2 m–3 day–1) was calculated considering DO variation with time, dCo2/dt (without aeration) and the biomass concentration present in the bioreactor, according to the following equation:
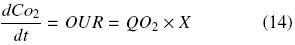
where QO2 represents the specific oxygen consumption (kg O2 kg DW–1 day–1) and X is the biomass concentration (kg DWm–3). DWcorresponds to dry weight of cells. Using a graph of CO2 vs. time, OUR (QO2 × X) is obtained calculating the slope of the strength line (Doran, 1995).
2.5 Maximum QO2 achievable in shake flask
The maximum oxygen consumption that A. indica cells can have in shake flasks with a particular OTRmax was calculated using the equation:

The maximum QO2 (achievable by oxygen transfer) vs. biomass was plotted for the three cases of shake flasks closers (plugged with silicone foam, cotton and aluminum foil). These values were compared with the QO2 measured in the bioreactor (without oxygen limitation).
2.6 Statistical analysis
The experiments were carried out in triplicate and each sample was analyzed twice. Mean values ± standard error are reported. Statistically significant differences between groups were evaluated with one–way analysis of variance and Fisher's least significant difference (LSD) procedure at the 95 % confidence level using Statgraphics for Windows 4.1.
3 Results and discussion
3.1 Oxygen transfer in shake flasks
OTRmax obtained with shake flasks covered with cotton and silicone foam was higher than that obtained with aluminum foil, 8 and 15 times, respectively (Table 1). So, resistance to oxygen transfer using silicone foam was lower than using aluminum foil and cotton plug (Fig. 2). The resistance of the closure represents 95.4 %, 60.7 % and 31.2 % of the total resistance with aluminum foil, cotton and silicone foam, respectively. In particular, with aluminum and cotton closures the main resistance to oxygen transfer is in the closure and not in the liquid gas interface. In any case, resistance in the closure cannot be neglected. The mass transfer occurring in shake flask, neglecting the closure resistance has been modeled (Maier et al., 2004); however, the data obtained in the present research indicate that this consideration may lead to significant errors in the calculations of oxygen transfer.
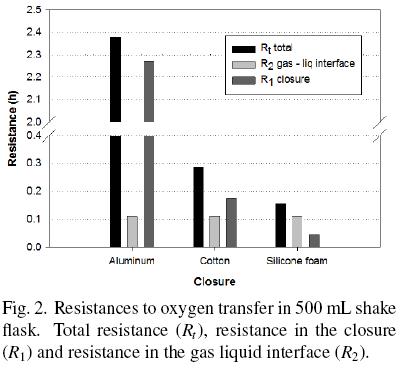
In this research, the dynamic gassing out method for estimate the oxygen transfer rate was used. There are other methods to determine the oxygen transfer in shake flasks which use non–invasive or invasive electrodes, approximation of steady state or non–steady state in the oxygen balance for the headspace in the flask, sulfite oxidation method, on–line monitoring system with oxygen sensor, etc. (Boon and Heijnen, 1998; Veglio et al., 1998; Anderlei et al., 2004; Anderlei et al., 2007; Amoabediny and Büchs, 2007; Deshpande and Heinzle, 2009). The method used to determine oxygen transfer rate can affect strongly the obtained value and each method has its advantages and disadvantages. So, the use of invasive electrodes can introduce a baffle effect but measure directly into de medium the oxygen concentration. Hansen et al. (2011) found a reduction of 13.7 % in OTR in a 250 mL shake flask (150 rpm, cotton as closure, 40 mL of sulphite sodium solution) using a non–invasive electrode. Amoabediny and Büchs (2007) found between 1.1 and 25 % longer OTR considering unsteady–state conditions in shake flasks with sulphite sodium solution as oxidation system. Although the results of these researchers contribute to improve the correct OTR determination in shake flasks, they used the sulphite oxidation as system, which is complex and strongly dependent of sulphite sodium and cobalt (used as catalyst) concentrations (Puskeiler et al., 2005; Poughon et al., 2003). Maier et al. (2004) found that OTR of a Pichia pastoris culture was 2.8 times OTRmax of the sulphite sodium system in shake flasks. Taking into account that the biomass presence can increase or decrease the oxygen transfer coefficient and this variation depend of cell concentration and extracellular compounds production (Garcia–Ochoa and Gomez, 2009) we decided to do the measurements in this study with a culture medium free of cells. Considering besides the main purpose of estimate the oxygen transfer coefficients and the relationships between the different resistances to oxygen transfer, we do not numerically compare our results with other researchers.
3.2 Effect of the type of closure on A. indica cell culture performance
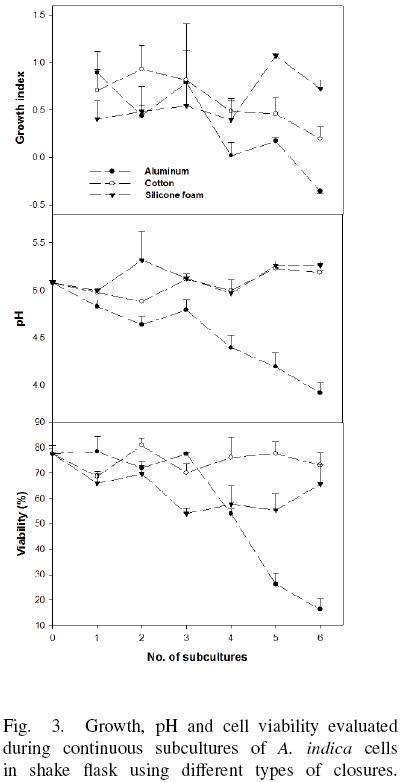
A. indica cell culture has positive growth index (GI) after of six subcultures using cotton and silicone foam, with pH variations between 4.9 and 5.3, and cell viability higher than 50% (Fig. 3). However, using aluminum foil the GI was zero after of the subculture 4 and was negative at subculture 6. In this case, the pH of the medium decreased from 5.1 to 4.0, and cell viability fall until 16 %. Cell suspension cultures with pH < 4.0 and cell viability lower than 25 % did not show cell growth further. Additionally, it was observed differences in aggregate after of 6 subcultures (Fig. 4). Cells cultured in flasks covered with aluminum foil produce aggregates with a diameter lower than 1 mm, whereas aggregates growing in flasks covered with cotton had a diameter between 3 and 5 mm, and aggregates obtained with silicone foam cap were the largest (reaching 10 mm).
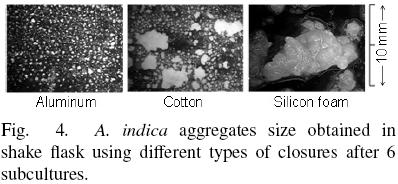
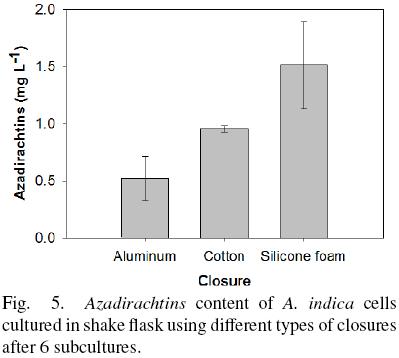
On the other hand, the content of Azadirachtins evaluated after 6 subcultures is shown in Fig. 5. The lowest production of Azadirachtins was observed using aluminum foil (0.52 ± 0.19 mg L –1) and the highest content was observed with silicone foam closure (1.51 ± 0.38 mg L The result was similar considering the Azadirachtins production by gram of biomass (0.06, 0.07 and 0.12 mg/g using aluminum, cotton plug and silicone foam closure, respectively). The level of Azadirachtins produced in the cultures in vitro is lower than that reported by the fruits of A. indica 5.13 mg g–1 DW (Prakash et al., 2005).
A. indica culture developed in Erlenmeyer flask covered with aluminum foil had the lower OTR value and cell culture present a reduction of their pH (Fig. 3). This phenomena may be a consequence of lactic acid accumulation produced by the cells as a result of hypoxic or anoxic conditions by the low oxygen availability (Geigenberger, 2003; Tadege et al., 1999). It is possible that A. indica cells under anoxia cannot withstand this pH reduction in the long term, and they lose viability and die. Then again, cells with low oxygen availability reduce their aggregates size. This result suggests that oxygen limitation stress produce a reduction of the aggregate size and could be similar to that observed in plant tissues (Potters et al., 2007). It was reported that plants exposed to abiotic stresses such as flooding, dryness and UV radiation stress induced variations in the cells (size and forms) in plant tissues.
The reduction of A. indica aggregates may be associated with a culture response to oxygen limitations. Kebler et al., (1999) proposed a relationship to know the maximum diameter for unlimited respiration throughout the aggregate. This report indicates that the diameter of the aggregate is proportional to the square root of the oxygen concentration at the surface, and different ajmalicine concentrations was found among the different aggregate sizes. The relationship between aggregate size and oxygen concentration is in agreement with our results. Higher OTRmax are related with a higher oxygen concentration in the liquid medium and allow larger aggregates to developed, as occurred with cultures growing in shake flasks using silicone foam closures. The higher content of Azadirachtins measured under this condition with a better oxygen supply or with cell differentiation, could lead to enhanced production of secondary metabolites (Zhao et al., 2003).
3.3 OUR of A. indica cultured in a bioreactor and comparison with QO2,max obtained in shake flask
In order to show that the poor growth and azadiracthtins production in shake flasks covered with aluminum foil (the lower OTR) could be related with a high oxygen consumption by A. indica cells, the OUR of cells was measured in a bioreactor without oxygen limitations. So then, A. indica cell culture had a consumption of 0.100 ± 0.016 kg O2 kg DW–1 day–1. The OUR for B. vulgaris cell culture was 0.027 ± 0.010 kg DW–1 day–1. These measurements were done using the same conditions and the results indicated that A. indica cells consume four times more oxygen than B. vulgaris (p < 0.05).
OTRmax values for the different closures (Table 1) were used to calculate the QO2,max in shake flasks using different closures (aluminum, cotton, silicone foam). QO2,max was plotted as a function of the biomass concentrations and this is showed in the Fig. 6. In the same figure, it is showed the QO2 measured in a culture developed in a bioreactor (horizontal lines), as a comparative condition without oxygen limitations. In this condition, for A. indica cultures QO2 was of 0.100 kg O2 kg DW–1 day–1, and for B. vulgaris was of 0.026 kg O2 kg DW–1 day–1. In shake flasks, QO2 decrease as the biomass increase and this behaviour is independent of the type of closure used. The QO2 profile of A. indica cultures developed in shake flaks with the different closures indicated that there are oxygen limitations when biomass concentrations increase. These oxygen limitations using aluminium foil closure was at 0.7 g DW L –1, for cotton was at 5.7 g DW L –1, and for silicone foam was with 10.4 g DW L –1. Doing a similar analysis with B. vulgaris, which can be cultivated for long terms in shake flasks, this specie presented oxygen limitations by biomass concentrations of 2.6 g DW L –1 with aluminium foil, 21.3 g DW L –1 with cotton, and 40.0 g DW L –1 with silicone foam. This consideration allows understand how B. vulgaris cell cultures could be developed in shake flasks with different covers, reaching high biomass concentrations, while A. indica cell had limitations to grow by oxygen limitations.

B. vulgaris does not lose viability when using aluminum foil as a close (Rodríguez–Monroy y Galindo, 1999) and consume only 25 % of oxygen that A. indica. These results agree with those reported by Geigenberger (2003) who showed that plant tissues can reduce oxygen consumption when external oxygen concentration decrease considerably or when oxygen concentration within the tissue falls even in well–oxygenated atmospheres. Plants can reduce the respiration rate, change their metabolism and protein degradation or modify their morphology to avoid internal anoxia. Only when oxygen concentration is close to zero, the cells turn on the mechanism to produce ATP in anaerobic conditions, using the lactate dehydrogenase and alcohol dehydrogenase enzymes (Geigenberger, 2003). Therefore, the lowest aggregate size and content of Azadirachtins observed in shake flasks covered with aluminum foil may be related to insufficient oxygen supply and a reduction in their metabolism.
It is necessary to analyze oxygen consumption of different plant species especially for the in vitro establishment of new plant cell cultures. If the plant cell culture has high oxygen consumption, the effect of low OTR in primary and secondary metabolism and its morphology should be studied because growth and secondary metabolism may be limited. For increasing the OTR in shake flasks, it may be necessary to use a closure with high oxygen transfer or modified other variables that affect the OTR (e.g., volume of culture medium in the flask or agitation speed).
Conclusions
OTRs of 0.07, 0.58 and 1.07 kg O2 m –3 day –1 were obtained using several closures of shake flask: aluminum, cotton and silicone foam, respectively. OTR lower affected A. indica cell culture in shake flasks (cell viability, pH medium, aggregate morphology and Azadirachtins) decreasing the cell growth and the Azadirachtins production. These results show the oxygen demand of this plant cell culture may be a limiting factor to grow in Erlenmeyer shake flasks.
Acknowledgments
This work was supported by CONACYT (grant 89321) and SIP–IPN (grant 20100401). F. Orozco–Sanchez is grateful to the Secretaría de Relaciones Exteriores (Mexico) for the doctoral fellowship awarded.
References
Amoabediny, G. and Büchs, J. (2007). Modelling and advanced understanding of unsteady–state gas transfer in shaking bioreactors. Biotechnology and Applied Biochemistry 46, 57–67. [ Links ]
Anderlei, T., Mrotzek, C., Bartsch, S., Amoabediny, G., Peter, C.P. and Büchs, J. (2007). New method to determine the mass transfer resistance of sterile closures for shaken bioreactors. Biotechnology and Bioengineering 98, 999–1007. [ Links ]
Anderlei, T., Zang, W., Papaspyrou, M. and Büchs, J. (2004). Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochemical Engineering Journal 17, 187–194. [ Links ]
Balaji, K., Veeresham, C., Srisilam, K. and Kokate, C. (2003). Azadirachtin, a novel biopesticide from cell cultures of Azadirachta indica. Journal of Plant Biotechnology 5, 121–129. [ Links ]
Boon, M. and Heijnen, J.J. (1998). Gas–liquid mass transfer phenomena in bio–oxidation experiments of sulphide minerals: A critical review of literature data. Hydrometallurgy 48, 187–204. [ Links ]
Büchs, J. (2001). Introduction to advantages and problems of shaken cultures. Biochemical Engineering Journal 7, 91–98. [ Links ]
Capataz–Tafur, J., Orozco–Sanchez, F., Vergara–Ruiz, R. and Hoyos–Sanchez, R. (2007). Efecto antialmentario de extractos de suspensiones celulares de Azadirachta indica sobre Spodoptera frugiperda J. E. Smith bajo condiciones de laboratorio. Revista Facultad Nacional de Agronomía, Medellín 60, 3703–3715. [ Links ]
Deshpande, R.R. and Heinzle, E. (2009). On line monitoring of oxygen in spinner flasks. Biotechnology Letters 31, 665–669. [ Links ]
Doran, P. (1995). Bioprocess Engineering Principles. Academic Press. Academic Press, London. [ Links ]
Garcia–Ochoa, F. and Gomez E. (2009). Bioreactor scale–up and oxygen transfer rate in microbial processes: An overview. Biotechnology Advances 27, 153–176. [ Links ]
Geigenberger, P. (2003). Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology 6, 247–256. [ Links ]
Gupta, A. and Rao, G. (2003). A study of oxygen transfer in shake flasks using a non–invasive oxygen sensor. Biotechnology and Bioengineering 84, 351–358. [ Links ]
Hansen, S., Kensy, F., Käser, A. and Büchs, J. (2011). Potential errors in conventional DOT measurement techniques in shake flasks and verification using a rotating flexitube optical sensor. BMC Biotechnology 11, 1–7. [ Links ]
Kebler, M., ten Hoopen, H.J.G. and Furusaki, S. (1999). The effect of the aggregate size on the production of ajmalicine and tryptamine in Catharanthus roseus suspension culture. Enzyme and Microbial Technology 24, 308–315. [ Links ]
Kieran, P.M., MacLoughlin, P.F. and Malone, D.M. (1997). Plant cell suspension cultures: some engineering considerations. Journal of Biotechnology 59, 39–52. [ Links ]
Lee, C.W.T. and Shuler, M.L. (1991). Different shake flask closures alter gas phase composition and ajmalicine production in Catharanthus roseus cell suspensions. Biotechnology Techniques 5, 173–178. [ Links ]
Maier, U., Losen, M. and Büchs, J. (2004). Advances in understanding and modeling the gas–liquid mass transfer in shake flasks. Biochemical Engineering Journal 17, 155–167. [ Links ]
Mantzouridou, F., Roukas, T. and Achatz, B. (2005). Effect of oxygen transfer rate on [beta]–carotene production from synthetic medium by Blakeslea trispora in shake flask culture. Enzyme and Microbial Technology 37, 687–694. [ Links ]
Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiology 15, 473–497. [ Links ]
Nikakhtari, H. and Hill, G.A. (2006). Closure effects on oxygen transfer and aerobic growth in shake flasks. Biotechnology and Bioengineering 96, 15–21. [ Links ]
Orozco–Saínchez, F. and Rodríguez–Monroy, M. (2007). Cultivos de células en suspensioín de Azadirachta indica para la produccioín de un bioinsecticida. Revista Mexicana de Ingeniería Química 6, 251–258. [ Links ]
Potters, G., Pastarnak, T., Guisez, Y. and Palme, K. (2007). Stress–induced morphogenic respondes: growing out of trouble? Trends in Plant Science 12, 98–105. [ Links ]
Poughon, L., Duchez, D., Cornet, J.F. and Dussap, C.G. (2003). kLa determination: Comparative study for a gas mass balance method. Bioprocess and Biosystems Engineering 25, 341–348. [ Links ]
Prakash, G.; Emmanuel, C.J. and Srivastava, A. K. (2005). Variability or Azadirachtin in Azadirachta indica (neem) and batch kinetics studies of cell suspension culture. Biotechnology and Bioprocess Engineering 10, 198 – 204. [ Links ]
Puskeiler, R.T. and Weuster–Bot, D. (2005). Combined sulfite method for the measurement of the oxygen transfer coefficient kLa in bioreactors. Journal of Biotechnology 120, 430438. [ Links ]
Rodríguez–Monroy, M., Trejo–Espino, J.L., Jiménez–Aparicio, A., Morante, M. and Trejo–Tapia, G. (2004) Evaluation of morphological properties of Solanum chrysotrichum cell cultures in a shake flask and fermentor and rheological properties of broths. Food Technology and Biotechnology 42, 153–158. [ Links ]
Rodríguez–Monroy, M. and Galindo, E. (1999). Broth rheology, growth and metabolite production of Beta vulgaris suspension culture: a comparative study between cultures grown in shake flasks and in a stirred tank. Enzyme and Microbial Technology 24, 687–693. [ Links ]
Schaaf, O., Jarvis, A.P., van der Esch, S.A., Giagnacovo, G. and Oldham, N.J. (2000). Rapid and sensitive analysis of Azadirachtin and related triterpenoids from Neem (Azadirachta indica) by high–performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. The Journal of Chromatography A 886, 89–97. [ Links ]
Suresh, S., Srivastava, V.C. and Mishra, I.M. (2009). Critical analysis of engineering aspects of shaken flask bioreactors. Critical Reviews in Biotechnology 29, 255–278. [ Links ]
Tadege, M., Dupuis, I. and Kuhlemeier, C. (1999). Ethanolic fermentation: new functions for an old pathway. Trends in Plant Science 4, 320235. [ Links ]
Veglio, F., Beolchini, F. and Ubaldini, S. (1998). Empirical models for oxygen mass transfer: a comparison between shake flask and lab–scale fermentor and application to manganiferous ore bioleaching. Process Biochemstry 33, 367–376. [ Links ]
Withers, L.A. (1985) Cryopreservation and storage of germplasm. In: Dixon RA (ed) Plant cell culture, a practical approach. IRL Press, Oxford, pp. 169–192. [ Links ]
Zhao, D., Huang, Y., Jin, Z., Qu, Z. and Lu, D. (2003). Effect of aggregate size in cell cultures of Saussurea medusa on cell growth and jaceosidin production. Plant Cell Reports 21, 1129–1133. [ Links ]














