Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.2 Ciudad de México Ago. 2011
Biotecnología
Morphological characterization of aerial hypahe and simulation growth of Fusarium solani under different carbon source for application in the hydrofobic VOCs biofiltration
Caracterización morfológica de hifas aéreas y simulación del crecimiento de Fusarium solani bajo diferentes fuentes de carbono para su aplicación en la biofiltración de COVs hidrofóbicos
A. Vergara–Fernández1,2*, S. Hernández1, J. San Martín–Davison2 and S. Revah3
1 Departamento de Ingeniería de Procesos e Hidráulica, Universidad Autónoma Metropolitana–Iztapalapa, Apdo. Postal 55–534, CP 09340, México DF, México.
2 Centro de Energías Renovables y Calidad Ambiental, Escuela de Ingeniería de Procesos Industriales, Facultad de Ingeniería, Universidad Católica de Temuco, Rudecindo Ortega 0259 Campus Norte, Casilla 15–D, Temuco, Chile. * Corresponding author. E–mail: avergara@uctemuco.cl Tel.+56–45–205684, Fax +56–45–205630.
3 Departamento de Procesos y Tecnología, Universidad Autónoma Metropolitana–Cuajimalpa, c/o IPH, UAM–Iztapalapa, Av. San Rafael Atlixco No. 186, 09340 México D. F., Mexico.
Received 28 of April 2011.
Accepted 27 of June 2011.
Abstract
This work presents the effect of different carbon sources (glycerol, 1–hexanol and n–hexane) over the morphology of the aerial hyphae of the filamentous fungus Fusarium solani for its application in the biofiltration of volatile organic compounds (VOCs). A mathematical model was developed and further verified that combines microscopic and macroscopic parameters describing the mycelial fungal growth. Image analysis of microcultures and culture in agar dishes was performed to determine the morphological parameters. Theresults show that the hydrophobic and volatile carbon rources modified the morphology of Fusarium solani, this is associated with thebettor utilization of the volatile carbon source. The main morphology changes observed with glycerol and n–hexane, were the reduction in both the hyphal diameter (from 2.99+0.29 μm to 2.01+0.35 μm) and the average hyphal length (from 603.8+48.3 μm to 280. 1+36.6 μm). These results indicate an increase in the transport area for the same amount of biomass as an adaptation response to increase the uptake of volatile hydrophobic substrates.
Keywords: filamentous fungi, morphology, fungal biofiltration, Fusarium solani.
Resumen
Este trabajo presenta el efecto de diferentes fuentes de carbono (glicerol, 1–hexanol y n–hexano) sobre la morfología de las hifas aéreas del hongo filamento Fusarium solani para su aplicación en la biofiltración de compuestos orgánicos volátiles (COVs). Un modelo matemático que combina parámetros microscópicos y macroscópicos que describen el crecimiento del micelio del hongo fue desarrollado y verificado. Análisis de imágenes de los microcultivos y cultivos en placas de agar fue realizado para determinar los parámetros morfológicos. Los resultados muestran que las fuentes de carbono hidrofóbicas y volátiles modifican la morfología de Fusarium solani, esto está asociado con la mejor utilización de la fuente de carbono volátil. Los principales cambios morfológicos observados con glicerol y n–hexano, fueron la reducción en ambos diámetros de las hifas (desde 2.99+0.29 μm a 2.01+0.35 μm) y la longitud promedio de la hifa (desde 603.8+48.3 μm a 280.1+36.6 μm). Estos resultados indican un aumento en el Área de transporte para la misma cantidad de biomasa como una respuesta de adaptación para aumentar la captación de los sustratos hidrofóbicos volátiles.
Palabras clave: hongos filamentosos, morfología, biofiltración fúngica, Fusarium solani.
1 Introduction
Although bacteria are the predominant organism in most of the reported applications in biofilters for volatile organic compounds (VOCs) elimination, the presence of fungi, has been reported frequently even in cases where the conditions were manipulated to avoid their growth (Auria et al. 2000; Agathos et al. 1997).
Reports of fungi isolated from biofilters have shown that these microorganisms can metabolize hydrocarbons as their sole carbon source and energy. Several investigations have reported that fungi can degrade a wide variety of VOCs at elimination capacities (EC) equal or greater than those observed in bacterial systems (García–Peña et al. 2001; Woertz et al. 2001; Qi et al. 2002). The hyphae, due to its small diameter, have a very large surface area compared to its volume, which facilitates the diffusion of nutrients and carbon sources (Vergara–Fernández et al. 2006). Vergara–Fernández et al. (2008) suggests that the aerial mycelium, which is in direct contact with the gas, could make degradation of hydrophobic compounds faster than in a bacterial biofilm.
One of the main problems when studying the kinetics of filamentous fungi are the multiple processes involved in mycelial morphogenesis such as germination, vegetative and aerial growth and sporulation. For this reason, the models based on macroscopic parameters generally include implicitly the microscopic morphological parameters.
Trinci (1974) and Steele and Trinci (1975) were the first to introduce the morphological concept for the interpretations of fungal growth. Twenty years later a mathematical model proposed by Nielsen (1993), described branching, tip growth and differentiation during growth in submerged culture. McIntyre et al. (2001) developed a mathematical model to estimate the fragmentation rate of Aspergillus nidulans in a mixed batch reactor.
Lopez–Franco et al. (1994) analyzed the effect on the diameter and the rate radial of colony in many filamentous fungus growths under different culture conditions in petri dishes containing potato dextrose/agar. The effect of glucose concentration in the morphology parameters of Aspergillus niger was investigated by Larralde–Corona et al. (1997). Mendonca et al. (2005) observed morphologic changes on the hyphae of Neurospora crassa when was exposed to benzenic compounds from plants, including cinnamic acid, coumaric acid, ferulic acid, caffeic acid, and cinnamic aldehyde.
The objectives of this study were to quantitatively determinate, by developing a mathematical model including both microscopic and macroscopic parameters, the effect of different carbon sources (glycerol, 1–hexanol and n–hexane) on the changes in the aerial hyphae morphology of Fusarium solani. Furthermore, it was intended to relate these changes with the performance of fungal biofilters.
2 Model development
The individual hyphal growth was estimated by adding the main hyphal length and the branching from the main hyphae (Pazouki and Panda, 2000). In the Fig. 1 it is shown a representation of a mycelium. The variation of hyphal length was calculated according to Eq.(1):
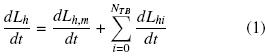

Where the second term is the main hyphal growth and the third term is the branching contribution.
The hyphal extension on a solid substrate can be described by the logistic expression (Okasaki et al. 980), and considering an average apical growth rate for each branch (urB) and a colony radial extension rate for the main hyphae, ur, the Eq. (2) was obtained:
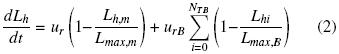
Where, NTB is the total number of branches in the individual hyphae. Then developing the summation and introducing the branching factor γi (Lhi = γiLh) and the fraction of the main hyphal length with respect to the individual total hyphal length λ (Lh,m= λLh), Eq. (3) was obtained:
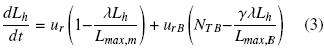
The radial extension rate of the colony for the main hyphae, Eq. (4), was represented according to Nielsen (1993) and McIntyre et al. (2001). Eq. (5) describes the branching growth rate according to Krabben et al. (1997):


Where, LAV is the average length of segments, defined according to Viniegra–González et al. (1993) (Eq. 6):

Where, Lh is the individual total hyphal length and NS the number of segment and equal NS = 2Nt,i—1, where Nt,i is the total number of tips in an individual hyphae. Then relating Lh and Nt,i the hyphal growth unit (G) was obtained according to Caldwell and Trinci (1973):

Replacing Eqs. (4) and (5) in Eq. (3) and considering a cylindrical hyphae and constant density biomass (Xh=Lhπ(Dh/2)2ρh), the individual biomass growth was obtained (Eq. 8). The total biomass and hyphal length were obtained according to Xh,Total=Xh · N0 and Lh,Total = Lh · N0, respectively.

Where, φ is the branching frequency, which was obtained integrating the section of the principal hyphae at the time t = τ, where τ is the time at the critical length of branching LC and equal to φ = 1/τ, according to Viniegra–González (2003) (Eq. 10) and μcalc is the specific rate determined according to Larralde–Corona et al. (1997) (Eq. 11).


3 Materials and methods
3.1 Microorganisms
Fusarium solani B1 has been reported previously by Vergara–Fernández et al. (2006), it was grown in agar plates and microculture. Its preservation, cultivation conditions and spore production were similar to reported by García–Peña et al. (2001).
3.2 Carbon sources and mineral medium
The mineral medium reported by Arriaga and Revah (2005) for fungi isolation, maintenance and cultivation was used. Three carbon sources were used: glycerol (0.7 g L–1) (Baker, 99.8%), 1–hexanol (0.7 g L–1) (Merck, 98%) and n–hexane (7 g m–3 in headspace) (Tecsiquim México, 95%).
3.3 Microcultures
Microcultures were realized in Petri dishes, where microscopic slides were placed on the top of glass bar and then were sterilized. Then agar blocks of 8 mm×1.5 cm×1.5 cm were placed on the top of the slide. The experimental system utilized is represented in the Fig. 2. Agar blocks were prepared with mineral medium, Noble Agar (30 g L–1 DifcoTM Becton Dickinson USA) and either glycerol or 1–hexanol as carbon source. Some dishes were prepared without carbon source and incubated in a closed chamber with n–hexane vapors. The four sides of the agar block were inoculated by direct puncture with a needle from a spore suspension (2 × 107 spores mL–1). Sterile cover slips were put on the top of the agar and then were cultivated at 30°C between 3 to 8 days, depending of the growth rate in each carbon source. A glycerin solution (5% v/v) was used to control the humidity in the dishes and to avoid the dehydration of agar. Four microcultures were performed for each carbon source.

3.4 Biomass growth in agar
Fungi were grown in Petri dishes at the same conditions that those in microcultures. Sterile hydrophilic membranes (Millipore, cellulose membrane 0.45 μm, 47 mm diameter) were placed on the top of the solidified agar and then were inoculated with 0.5 μL of a spore suspension containing 2 × 107 spores mL–1 in the center of the membrane and incubated at 30°C during 8 days. For the determination of the radial extension rate of the colony, Petri dishes without membranes were used and inoculated by puncture in the center of the dish and incubated for 8 days at 30°C (Esquivel–Viveros et al. 2009).
3.5 Analytical methods
The biomass grown on membranes was quantified by dry weight as reported by Vergara–Fernández et al. (2006) and González–Vazquez et al. (2011). Three dishes were used daily. Microcultures were analyzed in an Optic Microscopic (Nikon Optithop–2) equipped with a Leica DC 300 camera and a computer. The Leica IM50 (Version 4.0 Release 85) software was used for images acquisitions. The images were analyzed using the Image J v1.34s software (Wayne Rasband, National Institute of Health, USA). Pictures were digitalized to a resolution of 3132 x 2325 pixels.
For the hyphal diameter measurements LAV, LC, Lmax and LmaxB at least 20 hyphal tubes per microculture were measured. Radial growth was measured as the length increment of the colony every 24 h.
The experimental results were adjusted to the model proposed using the software Mathcad Plus.
4 Results and discussion
4.1 Morphology parameters
Table 1 shows the morphological parameters obtained for F. solani grown under different carbon sources in microcultures and Petri dishes. These results show that the carbon source has an effect over the radial rate extension of colony and important reductions between 22 to 54% were observed for the fungus grown in 1–hexanol and n–hexane respectively as compared to glycerol. These results can be attributed to the higher volatility and lower biodegradability of 1–hexanol and n–hexane. On the other hand, the rate of radial growth with 1–hexanol was higher than with n–hexane, probably due to its higher availability being less volatile and hydrophobic than n–hexane (Vergara–Fernández et al. 2006). The ur values obtained for F. solani grown in 1–hexanol were half to those obtained by Larralde–Corona et al. (1997) with Aspergillus niger to a glucose initial concentration of 10 g L–1, whereas Lopez–Franco et al. (1994) found 228 values which were 2.5 times larger than those obtained with glycerol with Fusarium culmorum grown in agar plates containing potato dextrose.

Furthermore, the different carbon sources have an effect on the morphological characteristics of F. solani, as seen in Fig. 3. The fungus grown in 1–hexanol and n–hexane presented a reduced diameter (about 30% compared with glycerol) which translates into an important increase in the transport area for the same amount of biomass. Vergara–Fernández et al. (2006) obtained similar results for the fungus F. solani grown on the same carbon sources, indicating that this difference in diameter predicts that for the same biomass, the exposed surface would be twice larger for 1–hexanol and n–hexane as compared to glycerol. In addition, Lmax increased 59% when the fungus was grown in n–hexane and 1–hexanol with respect to the value in glycerol. Similar result for LmaxB was obtained with an increase of 81% for n–hexane and 1–hexanol over glycerol.
The average hyphal diameters obtained with F. solani in this study was 1.5 times larger than those reported in the studies by López–Franco et al. (1994) with F. culmorum and Larralde–Corona et al. (1992) with Aspergillus niger, both grown in glucose.
The results obtained for LAV (Table 1) showed a decrease of 2.4 times for 1–hexanol and 2.2 times for n–hexane compared to glycerol, this behavior has consequences in the increment of the number of hyphae segments and therefore a greater number of ramifications, for the same hyphal length, it is also observed with an increased in the proportionality factor, γ. This factor increases 3 times when the fungus was grown with 1–hexanol and n–hexane as compared to glycerol. This effect in morphology is probably due to the longer ramifications; indicating a better contribution of the transport area. Rahardjo et al. (2002) also observed this contribution of aerial growth in the cellular respiration using Aspergillus oryzae.
4.2 Growth and simulation
Fig. 4 shows the growth of F. solani with three different carbon sources, the experimental results are denoted by the symbols, whereas the curve represents the model simulation with the morphological parameters condensed in Table 1. In all cases the model presents a good correlation with the fungal biomass obtain experimentally. These results support the feasibility of the model based on morphological parameters to represent the fungal biomass growth.

Fig. 4 shows that the biomass obtained for the fungi grown on 1–hexanol and n–hexane were similar, however it was 3 times smaller than the biomass grown on glycerol. The more abundant biomass produced from glycerol was attributed to its higher bioavailability, due to the lower solubility of n–hexane and 1–hexanol. On the other hand, the Fig. 4 show that the carbon source has an effect over the specific growth rate (μ) and important reductions between 25% (μ = 0.037 h–1) to 59% (μ = 0.029 h–1) were observed for the fungus grown in 1–hexanol and n–hexane respectively as compared to glycerol (μ = 0.046 day–1), similar result to that obtained for the radial extension of colony.
Fig. 5a) was constructed using the values of the Table 1. This figure shows when the fungus is grown in more hydrophobic and volatile carbon sources a greater number tips were obtained (120% more tips for 1–hexanol and 30% more with n–hexane as compared to glycerol). This indicate an important change in the morphology of aerial hyphae, considering that the biomass generated with glycerol was three time larger than that generated with 1–hexanol and n–hexane.

Values of NTotal obtained for n–hexane and 1–hexanol are related to the increase of the proportionally branching constant γ and the lower LAV. The fungus adapts to the carbon source by generating an increase in the length (see Table 1) and the amount of ramifications in the aerial hyphae. As result of these changes, in the aerial volume occupied by the fungus was increased (although with a lower apparent density) favoring the transfer contact between the more volatile carbon sources and the biomass. Vergara–Fernández et al. (2008) indicate that the growth of the fungal aerial hyphae enhances the EC of the hydrophobic VOCs, when compared with a bacterial biofilter, by increasing the transport area available, when the fungus is grown in a volatile and hydrophobic carbon source.
Fig. 5b) represents the correlation between the hyphal growth unit G estimated as a equivalent function to the amount of branching and Nt,i. It can be observed that for 1–hexanol and n–hexane G is constant between 2 and 3 number of tips for two days of growth approximately, while for glycerol G is constant between 5 and 6 number of tips, corresponding to approximately 3.5 days of growth.
These results show that it is possible to consider constant the average length of segments, LAV, in the experimental conditions, according to the relationship between the Eqs. (6) and (7) given by the equation G = (NS/Nt,i) LAV = ζLAV, for growth periods over 2 days for 1–hexanol and n–hexane and for more of 3 days for glycerol. This is valid when G is constant.
The G max obtained when the fungus was grown in glycerol was 1150 μm, whereas when it was grown in 1–hexanol and n–hexane was on average 400 μm. This decrease is related to a 2.3 times reduction in the LAV value from glycerol to 1–hexanol and n–hexane.
Conclusions
The results show that hydrophobic volatile carbon sources have a strong influence in inducing morphological changes in Fusarium solani that increase the aerial mycelium and the contact area, and the mass transfer, with the carbon source. These findings are relevant to explain the higher uptake rates that fungal biofilters have shown with respect to bacterial biofilters when treating hydrophobic volatile pollutants.
The mathematical model developed presents a good correlation with the fungal biomass obtains experimentally. The independent evaluation of the parameters allowed a small deviation with experimental data below 8% for the biomass growth with all carbon sources used. The results support the feasibility of using a model based on combining microscopic and macroscopic parameters describing the mycelial fungal growth to represent the fungal biomass growth.
Acknowledgements
The authors wish to thank the Dirección de Investigación de la Universidad Católica de Temuco–Chile and SEMARNAT–CONACyT México for the doctorate support of A. Vergara–Fernández. The support of CONACyT (Mexican Council for Science and Technology) for this project is acknowledged.
Notation

References
Agathos, S.N., Hellin, E., Ali–Khodja, H., Deseveaux, S., Vandermesse, F. and Naveau, H. (1997). Gas–phase methyl ethyl ketone biodegradation in a tubular biofilm reactor: microbiological and bioprocess aspects. Biodegradation 8, 251–264. [ Links ]
Arriaga, S. and Revah, S. (2005). Removal of n–hexane by Fusarium solani with a gas–phase biofilter. Journal Industrial Microbiology and Biotechnology 32, 548–553. [ Links ]
Auria, R., Frere, G., Morales, M., Acuña, M.E. and Revah, S. (2000). Influence of mixing and water addition on the removal rate of toluene vapors in a biofilter. Biotechnology and Bioengineering 68, 448–455. [ Links ]
Caldwell, Y. and Trinci, P.J. (1973). The growth unit of the mould Geotrichum candidum. Archivfur Mikrobiologie 88, 1–10. [ Links ]
Esquivel–Viveros, A., Ponce–Vargas, F., Esponda–Aguilar, P., Prado–Barragán, L.A., Gutiérrez–Rojas, M., Lye, G.J. and Huerta–Ochoa, S. (2009). Biodegradation of [bmim][PF6] using Fusarium sp. Revista Mexicana de Ingeniería Química 8(2), 163–168. [ Links ]
García–Peña, E.I., Hernandez, S., Favela–Torres, E., Auria, R. and Revah, S. (2001). Toluene biofiltration by the fungus Scedosporium apiospermum TB1. Biotechnology and Bioengineering 76, 61–69. [ Links ]
González–Vázquez, R., Azaola–Espinosa, A., Osorio–Revilla, G., Gallardo–Velázquez, T., Cruz–Victoria, T., Arana–Errasquin, R. and Rivera–Espinoza, Y. (2011). The effect of different carbon sources and salts in the production of naringinase by Aspergillus niger ATCC1015. Revista Mexicana de Ingeniería Química 10(1), 1–8. [ Links ]
Krabben, P., Nielsen, J. and Michelsen, M.L. (1997). Analysis of single hyphal growth and fragmentation in submerged cultures using a population model. Chemical Engineering Science 52(15), 2641–2652. [ Links ]
Larralde–Corona, C.P., López–Isunza, F. and Viniegra–González, G. (1992). A kinetic model for germ tube elongation of Aspergillus niger. Abstract 335. August 16–21. 9th International Biotechnology Symposium Crystal City, VA. [ Links ]
Larralde–Corona, C.P., López–Isunza, F. and Viniegra–González, G. (1997). Morphometric evaluation of the specific growth rate of Aspergillus niger grown in agar plates at high glucose levels. Biotechnology and Bioengineering 56(3), 287–294. [ Links ]
López–Franco, R., Bartnicki–García, S. and Bracker, C.E. (1994). Pulsed growth of fungal hyphal tips. Proceedings of the National Academy of Sciences USA 91(25), 12228–12232. [ Links ]
McIntyre, M., Dynesen, J. and Nielsen, J. (2001). Morphological characterization of Aspergillus nidulans: growth, septation and fragmentation. Microbiology 147, 239–246. [ Links ]
Mendonça, F., Yoshiko, C. and Said, S. (2005). Effect of benzene compounds from plants on the growth and hyphal morphology in Neurospora crassa. Brazilian Journal Microbiology 36, 190–195. [ Links ]
Nielsen, J. (1993). A simple morphologically structured model describing the growth of filamentous microorganisms. Biotechnology and Bioengineering 41, 715–727. [ Links ]
Pazouki, M. and Panda, T. (2000). Understanding the morphology of fungi. Bioprocess Engineering 22, 127–143. [ Links ]
Qi, B., Moe, W.M. and Kinney, K.A., (2002). Biodegradation of volatile organic compounds by five fungal species. Applied Microbiology and Biotechnology 58(5), 684– 689. [ Links ]
Okazaki, N., Sugama, S. and Tanaka, T. (1980). Mathematical model for surface culture of Koji mold. Journal of Fermentation Technology 58, 471–476. [ Links ]
Trinci, A.P.J. (1974). A study of the kinetics of hyphal extension and branch initiation offungal mycelia. Journal of General Microbiology 81, 225–236. [ Links ]
Rahardjo, Y.S.P., Weber, F.J., le Comte, E.P., Tramper, J. and Rinzema, A. (2002). Contribution of aerial hyphae of Aspergillus oryzae to respiration in a model solid–state fermentation system. Biotechnology and Bioengineering 78(5), 539–544. [ Links ]
Steele, G.C. and Trinci, A.P.J. (1975). Morphology and growth kinetics of hyphae of differentiated and undifferentiated mycelia of Neurospora crassa. Journal General Microbiology 91, 362368. [ Links ]
Vergara–Fernández, A., Van Haaren, B. and Revah, S. (2006). Phase partition of gaseous hexane and surface hydrophobicity of Fusarium solani when grown in liquid and solid media with hexanol and hexane. Biotechnology Letters 28, 2011–2017. [ Links ]
Vergara–Fernández, A., Hernández, S. and Revah, S. (2008). Phenomenological model of fungal biofilters for the abatement of hydrophobic VOCs. Biotechnology and Bioengineering 101(6), 1182–1192. [ Links ]
Viniegra–González, G., Saucedo–Castaneda, G., Loi pez–Isunza, F. and Favela–Torres, E. (1993). Symmetric branching model for the kinetics of mycelial growth. Biotechnology and Bioengineering 42(1), 1–10. [ Links ]
Viniegra–González, G. (2003). Producción de enzimas por Aspergillus. BioTecnología Revista Sociedad Mexicana Biotectología Bioengeniería 8(2), 18–30. [ Links ]
Woertz, J.R., Kinney, K.A. and Szaniszlo, P.J. (2001). A fungal vapor–phase bioreactor for the removal of nitric oxide from waste gas streams. Journal of the Air and Waste Management Association 51, 895–902. [ Links ]














