Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.1 Ciudad de México abr. 2011
Catálisis, cinética y reactores
Unusual oxidative pattern in thermo–oxidation of dry films of cholesterol
Patrón oxidativo inusual en la termo–oxidación de películas secas de colesterol
I.G. Medina–Meza1, M.T. Rodríguez–Estrada2, G. Lercker2, I. Soto–Rodríguez3 and H.S. García1*
1 UNIDA, Instituto Tecnológico de Veracruz, M.A. de Quevedo 2779, Veracruz, Ver. 91897, México.*Corresponding author. E–mail: hsgarcía@itver.edu.mx Tel.: +52–229–9341469, ext. 116; Fax: +52–229–934–5701, ext. 201.
2 Dipartimento di Scienze degli Alimenti, Università di Bologna, viole Fanin 40, 1–40127, Bologna, Italy.
3 Facultad de Bioanálisis, Universidad Veracruzana, and Facultad de Medicina, Universidad Cristóbal Colón, Veracruz, México.
Received 22 of November 2010.
Accepted 10 of February 2011.
Abstract
Formation of cholesterol oxidation products (COPs) during dry heating of cholesterol films at 150°C for 48 h was studied. A method based on aminopropyl solid–phase extraction (SPE), followed by GC–MS analysis was employed for the identification and quantification of COPs. Two kinetic runs were performed: 60 min and 48 h of treatment. In the short run (60 min), 7–ketocholesterol and 25–hydroxycholesterol were detected in similar amounts after 30 and 15 min, respectively. Oxysterols present in higher amounts in the long run (48 h) were 7β–hydroxycholesterol (12.6 mg/g) and 5,6a–epoxycholesterol (13.4 mg/g). However, compounds whose formation is related to enzymatic oxidation and/or irradiation were detected, as 6–ketocholestanol (3.38 mg/g) and 4β–hydroxycholesterol (2.04 mg/g). 25–hydroxycholesterol (0.12 mg/g) was found after only 1 h, reaching 0.99 mg/g after 48 h of treatment. The results suggest that due to the physical state of cholesterol, the oxidative pattern was changed, favoring unusual routes, such as formation of 4β–OH and 6–keto from breakage of the epoxide ring. Regarding 25–hydroxycholesterol, it is assumed that the solid state favored exposure of the side chain, rendering it more susceptible to oxidation.
Keywords: 6–ketocholestanol, 4β–hydroxycholesterol, 25–hydroxycholesterol, cholesterol oxidation, thermo–oxidation.
Resumen
Se estudió la formación de productos de oxidación del colesterol (POCs) durante calentamiento de una película seca de colesterol a 150°C. ha metodología empleada fue extracción en fase sólida (SPE), seguida por análisis en GC–MS para la identificación y cuantificación de los POCs. Se realizaron dos cinéticas, de 60 min y 48 h de tratamiento. En la cinética corta (60 min), se detectaron 7–cetocolesterol y 25–hidroxicolesterol en cantidades similares después de 30 y 15 min, respectivamente. Los oxiesteroles presentes en mayores cantidades en la cinética larga (48 h) fueron el 7β–hidroxicolesterol (12.6 mg/g) y el 5,6a–epoxicolesterol (13.4 mg/g). Sin embargo, se detectaron compuestos cuya formación está relacionada a la oxidación enzimática y/o por irradiación, como el 6–cetocolestanol (3.38 mg/g) y el 4β–hidroxicolesterol (2.04 mg/g). El 25–hidroxicolesterol (0.12 mg/g) fue encontrado después de solo 1 h, llegando a 0.99 mg/g después de 48 h de tratamiento. Los resultados sugieren que, debido al estado físico del colesterol, el patrón oxidativo se modifica, favoreciendo rutas inusuales, como la formación del 4β–hidroxicolesterol y 6–cetocolestanol por ruptura del anillo epóxido. Por lo que se refiere al 25–hidroxicolesterol se asume que el estado sólido favorece la exposición de la cadena lateral, siendo ésta entonces más susceptible a la oxidación.
Palabras clave: 6–cetocolestanol, 4β–hidroxicolesterol, 25–hidroxicolesterol, oxidación del colesterol, termo–oxidación.
1 Introduction
Cholesterol is an important biological compound that is commonly found in foods, such as eggs and dairy and meat products (Osada et al., 2000; Hur et al., 2007). Cholesterol can undergo oxidation during heating or by being subjected to UV light in the presence of oxygen (Chien et al., 1998, 2004). Cholesterol oxidation products (COPs or oxysterols) contain at least one additional functional group, such as hydroxyl, ketone or epoxide groups in the sterol nucleus and/or on the side chain of the molecule. To date, more than 60 COPs species have been identified (Hur et al., 2007), of which 8 are the most commonly studied for their presence in foods (Yen et al., 2010). Cholesterol oxides are present in the diet, particularly in foods with high cholesterol contents. The presence of COPs in a variety of foods has been studied extensively (Rodríguez–Estrada et al., 1997; Lee et al., 2006; Soto–Rodríguez et al., 2008). Procedures currently employed to extract, purify and quantify COPs have been described by several authors (Dutta & Appelqvist, 1997; Dutta & Savage, 2002; Tai et al., 1999).
Oxysterols exert several in vitro and in vivo biochemical activities of both physiological and pathological relevance (Schroepher, 2000; Ryan et al., 2005; Poli et al., 2009), and are now considered to be important mediators of cholesterol–induced effects. Compared to cholesterol, the presence of an additional oxygen group renders these compounds more polar and thus more soluble in aqueous solutions.
Most of the studies about the presence of oxysterols in foods, report and quantify COPs of the sterol nucleus, focusing principally on 7–ketocholesterol, 5,6–(α,β)–epoxycholesterol and 7–hydroxycholesterol. However, side chain COPs and other minor oxidative products seem to be more directly involved in human pathologies, even in small doses (Johnson et al., 1988).
Among the various COPs, 25–hydroxycholesterol (25–OH), which is formed by enzymatic oxidation of cholesterol in mammalian tissue (Ryan et al., 2005), has been reported as a potent inducer of apoptosis (Poli et al., 2009). Johnson et al. (1988) demonstrated that an intake of a single dose of 25–OH (1 μg/kg), administered by intragastric tube to rats, produced inhibition of liver HMG–CoA reductase at 3 or 16 h. A study performed by Adams et al. (2004) revealed that 25–OH inhibited cholesterol synthesis by different biological mechanisms that involved transcription factors that activate genes encoding enzymes of lipid synthesis.
6–ketocholestanol (6–keto) and 4–cholesten–3–one both cause a large increase in dipole potential of lipid membrane surface (Le Goff et al., 2007) and this could have an important effect in lipid–protein interaction. Very small quantities of 6–keto (1–10 ppb) have been reported in fresh and non–irradiated chicken meat (Hwang & Maerker, 1993).
Finally, 4β–hydroxycholesterol (4β–OH) is one of the quantitatively most important oxysterols in human circulation and is formed by the drug–metabolizing enzyme cytochrome P450 3A4 (CYP3A4) in liver and intestine (Bodin et al., 2002). The physiological role of 4β–OH is not known; in vitro experiments have shown that it activates the nuclear liver X receptor alpha (LXRα) (Wide et al., 2008). The existence of 4β–hydroxylated bile acids in humans suggests that this oxysterol can be metabolized in the liver (Bodin et al., 2002). To the best of our knowledge, there are no reports on the formation of 4β–OH and 6–keto during non–enzymatic oxidation of cholesterol.
Auto–oxidation of cholesterol has been studied under a wide variety of experimental conditions (Yen et al., 2010, Chien et al., 1998). Mechanisms by which COPs are produced during heating or UV illumination of cholesterol have been described (Maerker, 1987; Guardiola et al., 1997; Lee et al., 2006). In a process similar to that for lipid oxidation, initially a series of reactions that lead to formation of free radicals occur during heating of cholesterol; these radicals subsequently react to form COPs. Both lipids and cholesterol can undergo a series of free radical chain reactions to form peroxides and other degradation products (Smith, 1996).
Heat, pH, light, oxygen, water activity, and the presence of unsaturated fatty acids are reported to be the major factors that influence formation of COPs during food processing or storage (Lee et al., 2008); however, the physical state of cholesterol may significantly change the oxidative pattern of the molecule and the study on the formation of new reaction pathways should be pursued.
The aim of this work was to study the effect of the physical state on the oxidative pattern of thermo–oxidized cholesterol dry films. The analytical parameters evaluated were peroxide value (POV), total COPs and single COPs species by GC–MS, focusing particularly in A–ring and side–chain oxidation products.
2 Materials and methods
2.1 Reagents, solvents and standards
Chloroform, n–hexane, methanol, diethyl ether and anhydrous sodium sulfate, were supplied in reagent grade quality by Merck (Darmstadt, Germany). Acetone (AnalaR®) was purchased from BDH (VWR International Ltd., Leicestershire, UK).
Aminopropyl solid–phase extraction (SPE) cartridges (500 mg stationary phase/3 mL strata cartridges) were purchased from Phenomenex (Torrance, CA). Analytical grade chemicals were obtained from Sigma–Aldrich (St. Louis, MO). The COP standards supplied by Sigma–Aldrich were: Cholesta–5–en–3β–ol (cholesterol), cholesta–5–en–3β,19–diol (19–hydroxycholesterol, 19–OH, lup–20(29)–ene–3β,28–diol (betulin), cholesta–5–en–3β–ol–7–one (7–ketocholesterol, 7–keto), 5α,6a–epoxycholestan–3β–ol (5,6a–epoxycholesterol, 5,6a–epoxy), 5β,6β–epoxycholestan–3β–ol (5,6 –epoxycholesterol, 5,6β–epoxy), cholestan–3β,5a,6β–triol (cholestanetriol, triol), cholesta–5–en–3β,7a–diol (7a–hydroxycholesterol, 7a–OH), cholesta–5–en–3β,7β–diol (7β–hydroxycholesterol, 7β–OH), cholesta–5–en–3β,4β–diol (4β–hydroxy–cholesterol, 4β–OH), 5a–Cholestan–3β–ol–6–one (6–ketocholestanol, 6–keto), cholesta–5–en–3β,20a–diol (20a–hydroxycholesterol, 20–OH) and cholesta–5–en–3β,25–diol (25–hydroxycholesterol, 25–OH).
The sylilating mixture was prepared with pyridin, hexamethyldisilazane (HMDS) and trimethylchlorosilane (TMCS), all supplied by Carlo Erba (Milano, Italy); the silylating mixture was prepared using proportions of 5:2:1 (v/v/v).
2.2 Thermo–oxidation of cholesterol
Ten mg of cholesterol were placed into 10–mL open glass vials and heated in a convection oven at 150 ±2°C. This temperature was chosen because several foods are often deep–fried or baked at 150°C. The heating time varied from 0 to 60 min; samples were withdrawn at 0, 1, 3, 7, 15, 30 and 60 min and subsequently at 2, 3, 6, 12, 24, 36 and 48 h. The trials were carried out in duplicate. After heating, the vials were quickly cooled, capped and stored frozen until analyzed.
2.3 Determination of peroxide value (POV)
Peroxide values were determined as described by Shantha & Decker (1994). This protocol involves measuring the ability of peroxides to oxidize ferrous ions to ferric ions. A double beam UV–Visible spectrophotometer (Shimadzu model UV–1601, Kyoto, Japan) was employed to measure the absorbance at 500 nm. For the quantitative determination of POV, a Fe (III) standard calibration curve was prepared for the concentration range 0.1–5 μg/mL (r2= 0.999). Peroxide values were expressed as meq active O2/kg; the analyses were conducted in duplicate.
2.4 Gas chromatographic determination of cholesterol
The Perkin–Elmer Autosystem XL gas chromatograph (Norwalk, CT) employed was equipped with a split–splitless injector, and an FID. The chromatograph contained a Varian CP–SIL 5CB capillary column (30 m x 0.25 mm i.d. x 0.1 μm film thickness). The injector and detector were both set at 325°C. The oven temperature was programmed to proceed initially from 265 to 280°C at 0.5°C/min, then from 280 to 325°C at 4°C/min, and finally held at 325°C for 15 min. Helium was used as the carrier gas at a flow rate of 2.88 mL/min, the split ratio was 1:15, and the operating pressure was constant at 75 kPa.
Quantification of cholesterol and COPs was based on use of the response factor for each compound to the analytical technique; in addition, the response of the column, silylation and detector were considered.
2.5 Determination of cholesterol oxidation products
Purification of the COPs by NH2 SPE was conducted according to the method of Rose–Sallin et al. (1995). Samples of heated cholesterol were diluted in 1 mL of n–hexane:ethyl acetate (95:5, v/v), and applied to aminopropyl SPE columns which had previously been activated by adding 3 mL of hexane. The hexane was allowed to settle in the column. Purification was accomplished by adding first 6 mL of n–hexane:ethyl acetate mixture (95:5, v/v), followed by 10 mL of an n–hexane:ethyl acetate solution (90:10, v/v). Oxysterols were recovered by adding 10 mL of acetone, 50 μL of 19–OH as an internal standard and then evaporating the acetone solution to dryness under a stream of nitrogen.
The purified fraction was silylated with pyridine:TMCS:HMDS (5:2:1, v/v/v) at 40ºC for 20 min, dried under a nitrogen stream, and dissolved in 1 mL of n–hexane. Then 1 μL of the solution of silylated COPs was injected to the GC–MS. Peak identification of each COP was accomplished by comparing the peak retention times with those of true standards as well as to the corresponding mass spectra. The response factors of the COPs and cholesterol were evaluated with respect to the use of the internal standard 19–OH. All GC–MS data were processed with Turbochrom Navigator software v. 6.1.1.0.0:K20 (Pelkin–Elmer, Norwalk, CT).
2.6 GC–MS analysis of COPs
A fused silica column (30 m x 0.25 mm i.d. x 0.25 μm thickness) coated with 5% phenyl polysyloxane, ZB–5 Zebron, was used to separate eight major COPs, namely, 7α–OH, 5,6α– epoxy, 5,6β–epoxy, 7β–OH, 7–keto, 20–OH, 25–OH and triol, as well as the internal standard 19–OH. The injector was set at 325°C and the column temperature was programmed as follows: 250°C for 3 min, then heating from 250 to 280°C at 2°C/min, held at 280°C for 12 min, then heated from 280 to 320°C at 1.5°C/min, and held at 320°C for 3 min. Helium was used as the carrier gas at a flow rate of 0.37 mL/min; the split ratio was 1:15 and the pressure was 40.1 kPa.
The interface temperature for GC–MS was 210°C, with the electron multiplier voltage set at 960 V and the ionization energy at 70 V. Perfluorotributylamine was used for auto tune with m/z intensities at 69, 219, and 502.
The selected ion monitoring mode (SIM) was employed to detect 25–OH and 7–keto. The 25–OH was largely detected by the ion at (m/z 131) while 7–keto was detected by two other ions (m/z 472 and 367). In addition, COPs were also identified by comparing the retention times of unknown peaks to those of reference standards and chromatographic analyses were conducted with the added standards 7α–OH and 7β–OH at an m/z of 456; for 5,6α–epoxy and 5,6β–epoxy at m/z values of 321, 403, 404, 456 and 457; and triol, at m/z values of 367, 457, 472, 473 and 474. Quantification was accomplished using 19–OH as an internal standard. The calibration curve for each COP standard was obtained by plotting the concentration ratio against the area ratio.
Data were acquired in both total ion current (TIC) and single ion monitoring (SIM) modes. All GC–MS data were stored and processed with GCM Solution Software (Version 2.40; 1999–2005 Shimadzu).
2.7 Statistical analysis
Means and standard deviations were calculated using Statistica v. 6.0 Software (StatSoft, Tulsa, OK). One–way ANOVA was performed to evaluate the influence of time of exposure to heating at 150°C on peroxide value (POV) and COPs. Tukey's honest significant multiple comparison was used to determine statistical differences between samples at a 95% confidence level (p ≤ 0.05).
3 Results and discussion
3.1 Peroxide value
Cholesterol undergoes auto–oxidation by free radical chain reactions, although it is relatively stable in its pure state (Smith, 1996). The POV value provides a useful index for establishing the presence of primary oxidation products in cholesterol (Osada et al., 1993a); because peroxides constitute the primary identifiable oxidation products. Increases in the levels of cholesterol oxides and POVs have been reported to reflect heating for many other food systems, including lard and tallow (Yan & White, 1990), sunflower oil and corn oil (Choe & Min, 2006) and marine foods (Osada et al., 1993b).
Peroxide values for dry cholesterol films heated at 150°C for 60 min are presented in Table 1. POV increased with increasing time of exposure to heating, starting at 6.4 meq O2/kg after 1 min and increasing to 24.5 meq O2/kg after 60 min. These results are consistent with those of previous studies (Li et al., 1994).
In fact, Li et al. (1994) reported the existence of a significant linear correlation between the extent of oxidation of cholesterol and POV (r = 0.976, p < 0.05); Li's model system is based on the fact that cholesterol oxidation is strongly accelerated by auto–oxidation and heating. The data supports the hypothesis that oxidation of cholesterol proceeds in conjunction with auto–oxidation of coexisting unsaturated compounds.
By contrast, a significant decrease was observed in the POV of dry cholesterol films heated at 150°C for 48 h, starting from 24.7 meq O2/kg to reach a final value of 3.9 meq O2/kg (see Table (2) 3). After 6 h of heating, the rate of breakdown of the hydroperoxides exceeded the rate at which they were formed. This regime corresponds to the propagation phase of oxidation. In the advanced stage of oxidation (after 12 h), the POV level reached a plateau, indicating similar rates of formation and breakdown of hydroperoxides. During the evolution step the POV decreased from 24.7 at 1 h, to 3.9 meq/kg after 48 h. Our results agree with those reported by Ohshima et al. (1996). These workers related the accumulation of COPs and POVs to the total lipids present during grilling for up to 15 min, and subsequent refrigerated storage for up to 3 days. Based on these results, two main phenomena are observed: initially the POV rises significantly during extended periods of heating, but at relatively elevated temperatures eventually decreases during dry heating of thin films of cholesterol and its derivatives.

3.2 Oxidation of cholesterol during heating
In general, solid dry cholesterol exhibits high resistance to oxidation. It has been reported that cholesterol is relatively stable at 100°C; however, when the temperature was raised to 150°C, cholesterol became unstable (Smith, 1987). Furthermore, but paradoxically, it is possible that accumulation of oxidation products may have an inhibitory effect on the oxidation rate, as proposed by Kim & Nawar (1993). At temperatures approximately 20°C below the melting point of cholesterol (148.5°C), cholesterol remained in a solid state and the rate of oxidation was slow. Above this temperature, melting was observed, as was a sudden increase in the rate of oxidation.
Melting of heated cholesterol below its nominal melting point is probably a response of the melting point to the presence of trace amounts of oxidation products formed during heating to the melting point. After approximately 40 h of heating, when the sample was held at these temperatures it melted completely and the rate of oxidation decreased. It is not clear why such a notable decrease in the oxidation rate occurred, although the accumulation of COPs may have an inhibitory effect on the rate of oxidation (Smith, 1987).
Data for the percentage of cholesterol remaining after heating are displayed in Tables 1 and 2; the percentages of cholesterol remaining were 55.5 and 15.3 of the initial amount after heating for 60 min and 48 h, respectively. Osada et al. (1993a) noted that 40% of the cholesterol remained unchanged after dry cholesterol had been held at 150°C for 24 h. In a similar study, Chien et al. (1998) reported that 33.3% of the initial cholesterol remained after 30 min of heating. Kim & Nawar (1993) studied the effect of temperature and type of substrate on the oxidation of cholesterol. Cholesterol remained solid at 120°C, and less than 10% had been oxidized after 80 h of heating. At 150 and 180°C, the cholesterol was liquid and more than 80% of the initial cholesterol disappeared within 1 h of heating; 24.4% of the initial cholesterol remained after 30 min at 150°C. These data suggest that differences in heating conditions control the rate of oxidation of cholesterol.
3.3 Cholesterol oxidation products formed during heating
Ten COPs and the internal standard, 19–OH, were well resolved using the GC conditions described in Materials and methods. The corresponding retention times are depicted in Table 3. Because of potential possible interferences in identification of the COPs by the type of column (DB–5), the SIM mode was used for detection of 25–OH and 7–keto. The m/z values employed are displayed in Table 3. These values were identical to those reported previously by Lee et al. (2006). Visual examination of the GC chromatogram indicated that 7α–OH, 7β–OH, 5,6β–epoxy, 5,6α–epoxy, 4β–OH, 6–keto, triol, 7–keto and 25–OH were the main COPs produced during heating of cholesterol under our experimental conditions. The oxidation products with shortest retention time was 7α–OH (20.7 min) and the COPs with the longest retention time was 7–keto (32.1) (Table 3). The COPs formed in 60 min and 48 h are indicated in Tables 1 and 4, respectively.
Beginning with the short kinetic (60 min), the presence of COPs were detected at the 3 min of heat exposure, being the 7α–OH and 7β–OH the first compounds formed with 0.01 mg/g for each one, followed by 25–OH with the same amount at 15 min of treatment (Table 1). The results confirm the attack of free radicals in the C–7 and C–25 position to form C–7–OOH and C–25–OOH, which are reduced to 7–OH isomers (α, β) and 25–OH, respectively.
On the other hand, Table 4 shows that the COPs formed in greater quantities in the long run (48 h) were 5,6α–epoxy and 7β–OH, with 13.4 mg/g and 12.6 mg/g respectively, followed by 5,6β–epoxy (7.1 mg/g), 7α–OH (5.1 mg/g) and 6–keto (3.38 mg/g). In this study 20α–OH was detected only in traces.
5,6–epoxide isomers are formed as a result of auto–oxidation of cholesterol both in the crystalline state, solid, or aqueous dispersion and account for 6–7% of the oxidation products of the total mixture of oxysterols (Maerker, 1987). In this paper the formation of 5,6α–epoxy was 13.4 mg/g and 7.1 mg/g for 5,6β–epoxide, which represents 13% and 7% respectively of total oxysterols. The ratio of epoxides (α/β), in this study was 2:1; this result is in contrast to Hwang & Maerker (1993), who obtained a α/β relation of 1.3:1.
Earlier investigations emphasize on cholesterol oxides derivates of the B–ring oxidation where the A–ring retains its original β–hydroxyl function: products included the isomeric 7 diols, the isomeric 5,6–epoxides and the 7–ketocholesterol. The formation of COPs in positions other than C–7 during thermo–oxidation has been poorly addressed, especially the 4β–OH and 6–keto. In contrast, in the present study we focused in compounds not only of C–7 but also where the A–ring has undergone oxidation. According to Maerker & Jones (1993), the 3β–hydroxy function of cholesterol could be oxidized to a 3–keto function, and the 5,6 double bond could move to the 4,5 position, in conjugation with the carbonyl group.
In general, as the heating time increased, the levels of most COPs followed an increasing trend. The triol was formed only after 2 h (0.27 mg/g). The formation of triol is most likely caused by hydrolysis of either the 5,6α–epoxy or 5,6β–epoxy form (Maerker & Jones, 1992).
There are several studies on the biological functions and pathological effects of both compounds but there are no reports on their formation during thermal oxidation. We found that 6–keto forms after 2 h of heat treatment with 0.26 mg/g, which increased linearly up to 3.38 mg/g at the end of treatment. Fig. 1 confirms the identity of this compound, showing the full mass spectra of the GC peak revealed at 31.5 min in a standard injection (A) and cholesterol sample after 48 h of treatment (B).
On the other hand, formation of 4β–OH started after 2 h of heating with 2.97 mg/g, and remained almost stable until 36 h, when it decreased to 2.04 mg/g at 48 h of treatment.
We hypothesize that under these conditions of treatment and especially considering the physical state of sample, minor pathways of cholesterol degradation were favored, such as the oxidation of the A–ring to give 4β–OH, and the opening of 5,6–epoxide ring resulting in the formation not only of triol but also of 6–keto. More research is suggested on the route of formation of these compounds during thermo–oxidation and kinetic modeling.
The levels of side–chain COPs and triol observed in the present study are somewhat different from those reported by previous investigators. Measurable amounts of 25–OH (0.042 mg/g) were found after only 30 min of heating; 7–keto (0.017 mg/g) was produced simultaneously. It is remarkable, however, that when cholesterol is subjected to dry heating, large quantities of 25–OH were produced relative to the 7–keto COP (see Fig. 2).
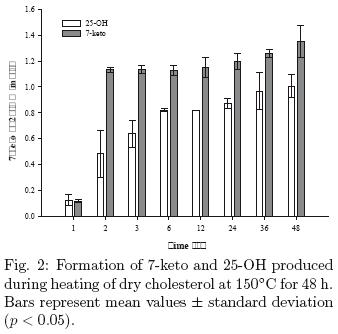
These results are in marked contrast with the results reported by Kim & Nawar (1993), who found only traces of side–chain derivatives (20–OH and 25–OH) and triols during dry–state oxidation at different temperatures (110–180°C) and heating times (0–80 h). Similarly, the results of this study are in agreement with Chien et al. (1998) with respect to the oxysterols derived from ring B, but do not concur with the formation of 25–OH, as during the heating of cholesterol in a model system to 150°C for 30 min, no side–chain COPs were detected.
At low temperatures, allylic C–7 and ternary C–25 radicals are formed initially in solid cholesterol; both radicals react readily with oxygen to give the corresponding peroxyl radicals. However, only the C–7 and 7–peroxyl radicals are stable at room temperature (Smith, 1987). Auto–oxidation of side–chain products usually occurs in dry solid cholesterol, but not in dispersions or solutions (Maerker & Jones, 1992).
Oxidative attacks at tertiary C–20 and C–25 positions generate 20–OOH and 25–OOH, respectively. These hydroperoxides are further reduced to 20–OH and 25–OH, which are more stable and can remain for 6 months during continuous heating at 100°C (Smith, 1987; Tai et al., 1999). The initiation reaction leading to formation of 25–OH is indicated by an electron spin resonance signal of a tertiary carbon–centered C–25 radical; this signal reveals that the tertiary C–25 carbon in cholesterol is the most reactive site for oxidation to a free radical. In fact, 25–hydroperoxide (25–OOH) is considered to be the third most prevalent peroxide auto–oxidation product from cholesterol (Smith, 1991). The C–25 radical may form directly from cholesterol by a primary hydrogen abstraction reaction similar to that reported at C–7, but it could also be formed by a radical transfer reaction involving a radical already formed by reaction with cholesterol. The reason why these changes are so destructive is that the hydroperoxide can break down to give either two free radicals (an alkoxyl and a hydroxyl radical), or it can generate a peroxy free radical, a hydroxyl free radical and water (Choe & Min, 2006).
The peroxyl radical may abstract a proton from C–25 according to the following scheme:
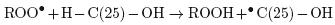
A possible demonstration of a free–radical initiated oxidation at the allylic C–25 of, not only cholesterol, but also 25–OH, would facilitate the development of rapid screening methods for analysis of cholesterol oxidation in food products.
The study of 25–OH in a model system may have biological significance. 25–OH could be an initiating oxidant in lipid peroxidation events associated with generation of  ions; 25–OH is usually formed endogenously from exogenous cholesterol and not from auto–oxidation of cholesterol (Johnson et al, 1988). Consequently, these oxidative effects could also occur in vivo. This possibility indicates a need for further studies to evaluate possibly significant effects of side–chain COPs leading to oxidative damage of biological tissues.
ions; 25–OH is usually formed endogenously from exogenous cholesterol and not from auto–oxidation of cholesterol (Johnson et al, 1988). Consequently, these oxidative effects could also occur in vivo. This possibility indicates a need for further studies to evaluate possibly significant effects of side–chain COPs leading to oxidative damage of biological tissues.
Similarly to what is assumed for the 6–keto and 4β–OH, the physical state of cholesterol and the crystalline arrangement appears to play an important role in determining the formation of 25–OH.
Abendan & Swift (2002) in a topographic study of cholesterol crystals using Chemical Force Microscopy (CFM) found that when cholesterol is in the monohydrate state, polar groups are mainly exposed (C–3, and double bond C–5/C–6). Conversely, in the case of anhydrous cholesterol, exposure of the aliphatic chain is favored (C–25). According to the type of dry film formed, we propose that the anhydrous solid form was prevalent in our samples. This could favor the oxidation of the side chain, and thus explains the abundant presence of 25–OH, which disagree with most of previous studies that oxidize cholesterol in solution or food matrix.
Conclusions
Thermo–oxidation of cholesterol dry films in a model system was studied. The results of the present work confirm the labile nature of solid cholesterol to oxidation by dry heat; the primary reaction pathway during heating probably involves free radical–mediated chain reactions. Under the experimental conditions tested in our experiments, 7–ketocholesterol was the predominant COP; however, other molecular processes occur in parallel leading to different oxidative patterns. A–ring COP like 4β–OH and different epoxide derivatives as 6–keto were found, despite the fact that previous authors indicate that they are usually a product of enzymatic oxidation and irradiation. In addition, measurable amounts of 25–OH were detected, a side–chain COP scarcely found in cholesterol auto–oxidation. We attribute these results primarily to the physical state of sample, which would favor both the exposure of the aliphatic chain to oxidation and a different behavior on epoxide breakage.
Given the potential of these COPs in biological and pathological fields, more attention should be paid to the quantification of these compounds in either model systems and processed foods.
Acknowledgements
The authors acknowledge the financial support of the National Council for Science and Technology of Mexico (CONACyT) through the grant 48086/A.
References
Abendan, R.S. and Swift, J.A. (2002). Surface characterization of cholesterol monohydrated single crystal by chemical force microscopy. Langmuir 18, 4847–4853 [ Links ]
Adams, C.M., Reitz, J., De Brabander, J.K., Feramisco, J.D., Li, L., Brown, M.S. and Goldstein, J.L. (2004). Cholesterol and 25–hydroxycholesterol inhibit activation of SREBPs by different mechanism, both involving SCAP and Insigs. The Journal of Biological Chemistry 279, 52772–52780. [ Links ]
Bodin, K., Andersson, U., Rystedt, E., Ellis, E., Norlin, M., Pikuleva, I., Eggertsen, G., Björkhem, I. and Diczfalusy, U. (2002). Metabolism of 4β–hydroxycholesterol in humans. The Journal of Biological Chemistry 276, 38685–38689. [ Links ]
Chien, J,T., Wang, H.C. and Chen, B.H. (1998). Kinetic model of the cholesterol oxidation during heating. Journal of Agricultural and Food Chemistry 46, 2572–2577. [ Links ]
Chien, J.T., Huang, D.Y. and Chen, B.H. (2004). Kinetic studies of cholesterol oxidation as inhibited by stearylamine during heating. Journal of Agricultural and Food Chemistry 52, 7132–7138. [ Links ]
Choe, E. and Min, D.B. (2006). Chemistry and reactions of reactive oxygen species in foods. Critical Reviews in Food Science and Nutrition, 46, 1–22. [ Links ]
Dutta, P.C. and Appelqvist, L.A. (1997). Studies on phytosterol oxides I: Effect of storage on the content in potato chips prepared in different vegetable oils. Journal of the American Oil Chemists' Society 74, 647–657. [ Links ]
Dutta, P.C. and Savage, G.P. (2002). Formation and content of phytosterol oxidation products in foods. In: Cholesterol and Phytosterol Oxidation Products. Analysis, Occurrence and Biological Effects. (F. Guardiola, P.C. Dutta, R. Codony and G. P. Savage, eds.), Pp. 319–334. Ed. AOCS Press, Champaign, IL. [ Links ]
Guardiola, F., Codony, R., Rafecas, M., Grau, A., Jordan, A. and Boatella, J. (1997). Oxysterol formation in spray–dried egg processed and stored under various conditions: Prevention and relationship with other quality parameters. Journal of Agricultural and Food Chemistry 45, 2229–2243. [ Links ]
Hur, S.J., Park, G.B. and Joo, S.T. (2007). Formation of cholesterol oxidation products (COPs) in animal products. Food Control 18, 939–947. [ Links ]
Hwang, K.T. and Maerker, G. (1993). Determination of 6–ketocholestanol in unirradiated and irradiated chicken meats. Journal of the American Oil Chemists' Society 70, 789–792. [ Links ]
Johnson, K.A., Montano, R.M., Morrow, C.J. and Scallen, T.J. (1988). In vivo formation of 25–hydroxicholesterol following a dietary cholesterol challenge. FASEB Journal 2, A580. [ Links ]
Kim, S.A. and Nawar, W.W. (1993). Parameters influencing cholesterol oxidation. Lipids 28, 917–922. [ Links ]
Le Goff, G., Vitha, M.F. and Clarke, R.J. (2007). Orientation polarisability of lipid membrane surface. Biochimica et Biophysica Acta 1768, 562–570. [ Links ]
Lee, H.W., Chien, J.T. and Chen, B.H. (2006). Formation of cholesterol oxidation products in marinated foods during heating. Journal of Agricultural and Food Chemistry 54, 4873–4879. [ Links ]
Lee, H.W., Chien, J.T. and Chen, B.H. (2008). Inhibition of cholesterol oxidation in marinated foods as affected by antioxidants during heating. Journal of Agricultural and Food Chemistry 108, 234–244. [ Links ]
Li, N., Ohshima, T., Shozen, K., Ushio, H. and Koizumi, C. (1994). Effects of the degree of unsaturation of coexisting triglycerols on cholesterol oxidation. Journal of the American Oil Chemists' Society 71, 623–627. [ Links ]
Maerker, G. (1987). Cholesterol autoxidation–Current status. Journal of the American Oil Chemists' Society 64, 388–392. [ Links ]
Maerker, G. and Jones, K.C. (1992). Gamma–irradiation of individual cholesterol oxidation products. Journal of the American Oil Chemists' Society 70, 255–259. [ Links ]
Maerker, G. and Jones, K.C. (1993). A–ring oxidation products from γ–irradiation of cholesterol in liposomes. Journal of the American Oil Chemists' Society 69, 451–455. [ Links ]
Osada, K., Kodama, T., Cui, L., Yamada, K. and Sugano, M. (1993a). Oxidation of cholesterol by heating. Journal of Agricultural and Food Chemistry 41, 1198–1202. [ Links ]
Osada, K., Kodama, T., Cui, L., Yamada, K. and Sugano, M. (1993b). Levels and formation of oxidized cholesterols in processed marine foods. Journal of Agricultural and Food Chemistry 41, 1893–1898. [ Links ]
Osada, K.S., Hoshina, S., Nakamura, S. and Sugano, M. (2000). Cholesterol oxidation in meat products and its regulation by supplementation of sodium nitrite and apple polyphenol before processing. Journal of Agricultural and Food Chemistry 48, 3823–3829. [ Links ]
Ohshima, T., Shozen, K.I., Ushio, H. and Koizumi, C. (1996). Effects of grilling on formation of cholesterol oxides in seafood products rich in polyunsaturated fatty acids. Lebensmittel–Wissenschaft und–Technologie 29, 94–99. [ Links ]
Smith, L.L. (1992). The oxidation of cholesterol. In: Biological Effects of Cholesterol Oxides, (S.K. Peng and R.J. Morin, eds.), Pp. 7–31. CRC Press, Boca Raton, FL. [ Links ]
Poli, G., Sottero, B., Gargiulo, S. and Leonarduzzi, G.(2009). Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Molecular Aspects in Medicine 30, 180–189. [ Links ]
Rodríguez–Estrada, M.T., Penazzi, G., Caboni, M.F., Bertacco, G. and Lercker, G. (1997). Effect of different cooking methods on some lipid and protein components of hamburgers. Meat Science 45, 365–375. [ Links ]
Rose–Sallin, C., Hugget, A.C., Boset, J.O., Tabacchi, R. and Fay, I.B. (1995). Quantification of cholesterol oxidation products in milk powders using [2H7] cholesterol to monitor cholesterol autoxidation artifacts. Journal of Agricultural and Food Chemistry 43, 935–941. [ Links ]
Ryan, L., O'Callaghan, Y.C. and O'Brien, N.M. (2005). Oxidized products of cholesterol: Their role in apoptosis. Current Nutrition in Food Science 1, 41–51. [ Links ]
Shantha, N.C. and Decker, E.A. (1994). Rapid, sensitive, iron–based spectrophotometric methods for determination of peroxide values of food lipids. Journal of the AOAC International 77, 421–424. [ Links ]
Schroepher, G.J. (2000). Oxysterols: modulators of cholesterol metabolism and other processes. Physiology Reviews 80, 361–554. [ Links ]
Smith, L.L. (1987). Cholesterol autooxidation. Chemistry and Physics of Lipids 44, 87–125. [ Links ]
Smith, L.L. (1996). Review of progress in sterol oxidation: 1987–1995. Lipids 31, 453–488. [ Links ]
Soto–Rodríguez, I., Campillo–Velázquez, P.J., Ortega–Martínez, J., Rodríguez–Estrada, M.T., Lercker, G. and García, H.S. (2008). Cholesterol oxidation in traditional Mexican dried and deep–fried food products. Journal of Food Composition and Analysis 21, 489–495. [ Links ]
Tai, C.Y., Chen, Y.C. and Chen, B.H. (1999). Analysis, formation and inhibition of cholesterol oxidation products in foods: An overview (part I). Journal of Food and Drug Analysis 7, 243–257. [ Links ]
Wide, K., Larsson, H., Bertilsson, L. and Diczfalusy, U. (2008). Time course of the increase in 4β–hydroxycholesterol concentration during carbamazepine treatment of paedriatic patients with epilepsy. British Journal of Clinical Pharmacology 65, 708–715. [ Links ]
Yan, P.S. and White, P.J. (1990). Cholesterol oxidation in heated lard enriched with two levels of cholesterol. Journal of the American Oil Chemists' Society 67, 927–931. [ Links ]
Yen, T.Y, Inbaraj, B.S., Chien, J.T. and Chen, B.H. (2010). Gas chromatography–mass spectrometry determination of conjugated linoleic acids and cholesterol oxides and their stability in a model system. Analytical Biochemistry 400, 130–138. [ Links ]














