Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.1 Ciudad de México abr. 2011
Biotecnología
The effect of different carbon sources and salts in the production of naringinase by Aspegillus niger ATCC1015
El efecto de diferentes fuentes de carbono y sales en la producción de naringinasa por Aspergillus niger ATCC1015
R. González–Vázquez1, A. Azaola–Espinosa2, G. Osorio–Revilla1, T. Gallardo–Velazquez1, T. Cruz–Victoria1, R. Arana–Errasquin1 and Y. Rivera–Espinoza1*
1 Departamento de Graduados e Investigación en Alimentos, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpio y Plan de Ayala, C.P. 11340, México City, México. *Corresponding author. E–mail: yrivera@encb.ipn.mx, espinoza4@hotmail.com Tel. (55) 5729–6000 ext 62464, Fax: (55) 5729–6000 ext 62346.
2 Universidad Autónoma Metropolitana. Departamento de Sistemas Biológicos. Calzada del Hueso, 1100, C.P. 04960, México City, México
Received 2 of October 2010.
Accepted 3 of January 2011.
Abstract
The effect of naringin, molasses, and rhamnose, as well as calcium (Ca2+) and magnesium (Mg2+) salts on the production of naringinase by Aspergillus niger ATCC1015 was studied. The mycelial weight, protein production, and naringinase activity were quantified. The constants Vmax and Km were determined by Lineweaver–Burk. ft was found that molasses compared with the other carbon sources and the addition of calcium carbonate favored the growth of the fungus but not the production of protein or the enzyme activity. Additionally, it was found that naringin promoted 8–fold more production of protein (5684 ± 784 μg·mL–1 ) than the other carbon sources studied if Ca2+and Mg2+were added. On the other hand, rhamnose with no added salts produced 2–fold more enzyme activity than other treatments (2290 ± 157 U·mg–1). Finally, it was found that its kinetic characteristics were Vmax=13.24 U·mg–1 and Km=1.6 mM, compared with the activity of a commercial naringinase from P. decumbens (Vmax=3.96 U·mg–1 and Km=2.5 mM).
Keywords: naringinase activity, Aspergillus niger, carbon sources.
Resumen
En el presente trabajo se estudió el efecto de las diferentes fuentes de carbono naringina, melaza y ramnosa, así como el efecto de la adición de las sales de calcio (Ca2+) y magnesio (Mg2+) en la producción de naringinasa por Aspergillus niger ATCC1015. Se cuantificaron el peso del micelio, la producción de proteína y la actividad de naringinasa. Las constantes Vmax y Km fueron determinadas utilizando el modelo de Lineweaver–Burk. Se encontró 27 que la adición de melaza con Ca2+ favorece el crecimiento del hongo sobre las otras fuentes de carbono no así la producción de proteína o la actividad enzimática. Además, se encontró que la producción de proteína fue 8 veces mayor (5684 ± 784 μg·mg–1) cuando se adicionó naringina con sales de Ca+2 y Mg+2. Por otra parte, la actividad enzimática fue 2 veces mayor cuando se adicionó ramnosa sin sales (2290 ± 157 U·mg–1). Finalmente, las características cinéticas determinadas fueron Vmax=13.24 U·mg–1 y Km=1.6 mM, con respecto a la actividad enzimática de la naringinasa comercial de P. decumbens (Vmax=3.96 U·mg–1 and Km=2.5 mM).
Palabras clave: actividad de naringinasa, Aspergillus niger, fuentes de carbono.
1 Introduction
Naringin (4,5,7–trihydroxyflavonone 7–rhamnoglucoside) is the main bitter flavonone glycoside. Its concentration is high in unripe fruit and decreases as fruit ripens ( ekero
ekero lu et al., 2006). The presence of naringin has been a major limitation on the commercial acceptance of citrus fruit juices. To improve the sensory properties and to stabilize finished fruit juice, the naringin level can be decreased by different technologies such as adsorptive debittering, chemical methods, and treatments with ion–exchange resin which have several limitations such as preparative steps on the juices and unfavorable changes on the sensory properties and nutritional value (Del Nobile et al., 2003).
lu et al., 2006). The presence of naringin has been a major limitation on the commercial acceptance of citrus fruit juices. To improve the sensory properties and to stabilize finished fruit juice, the naringin level can be decreased by different technologies such as adsorptive debittering, chemical methods, and treatments with ion–exchange resin which have several limitations such as preparative steps on the juices and unfavorable changes on the sensory properties and nutritional value (Del Nobile et al., 2003).
Naringinase is an enzyme which hydrolyses naringin due to its α–L–rhamnosidase and β–glucosidase activities. The α–L–rhamnosidase (EC 3.2.40) activity hydrolyses naringin into rhamnose and prunin [trihydroxyflavonone–7–glucoside], which bitterness is less than one–third that naringin. The activity of the rhamnosidase has been reported by some authors as the most important (Manzanares et al., 2001; Puri and Kalra, 2005). Prunin can be further hydrolysed by the β–glucosidase component (EC 3.2.1.21) into tasteless naringenin [4',5,7–trihydroxyflavonone] and glucose (Zverlov et al., 2000; Birgisson et al., 2004). Although naringinases are not common enzymes, they have become biotechnologically important due to their role in the manufacture of rhamnose and the preparation of naringenin and prunin (Vila Real et al., 2007).
Naringinase has been isolated from seeds, animal tissues, plants, and microorganisms (Gallego et al., 2001). However, for reasons of availability, only processes based on microbial naringinases are practicable (Puri et al., 2005). Naringinase is secreted by fungal species of Aspergillus and Penicillium (Norouzian et al., 2000) and the naringinase–producing Penicillium decumbens is commercially available (Puri and Kalra, 2005); however, there are few reports on its production, most of them are either guarded secrets of the industry or are patented (Puri et al., 2005). Production of naringinase depends on the inductor or carbon source given to the microorganism. Aspergillus niger is considered the best producer of extra–cellular naringinase (Kishi, 1955). It has been reported that metallic ions such as Ca2+ and Mg2+ contribute to the production of the enzyme but are not indispensable (Puri et al., 2005; Gallego et al., 2001).
Studies related to the culture conditions for the production process are controversial. Aspergilli species are eminent sources for the production of glycosidase activities in addition, the production of naringinase has been extensively studied in Aspergillus niger, in which the enzyme is extracellularly excreted (Chang et al., 2011). Commercial naringinase shows some limitations since it hydrolyses naringin only partially (Puri and Kalra, 2005). Due to one study explored 96 strains establishing Aspergillus niger as the best producer of naringinase (Kishi, 1955), the strain of Aspergillus niger ATCC 1015 was tested in order to improve upon hydrolysis of naringin. Furthermore, there is no literature available concerning to the production of naringinase by this Aspergillus niger producer of citric acid. In the present work in order to find an enzyme with higher activity and affinity, the effect of molasses, naringin and rhamnose, as well as, CaCO3 and MgCO3 on the protein production and the activity of naringinase were evaluated. Due to Vmax and Km reported in several articles have been evaluated using different substrate or under different conditions, in the present study were compared to the ones shown by Aspergillus niger ATCC 1015 versus a comercial naringinase from P. decumbens.
2 Materials and methods
2.1 Chemicals
Naringin, commercial naringinase (Penicillium decumbens), glucose rhamnose, malt extract and Sabouraud agar were obtained from Sigma, St. Louis, USA.
Molasses were obtained from a San Miguelito sugar mill in the Mexican State of Veracruz. All other reagents used were of analytical grade.
2.2 Microorganism
Aspergillus niger ATCC 1015 was obtained from a microbial culture collection, Centro de Investigación y de Estudios Avanzados (Mexico) and maintained on Sabouraud medium at 5ºC. This microorganism is reported as citric acid and antigen producer (CDBB: 177). The strain was cultured in order to obtain the amount required for the fermentations.
2.3 Preparation of the A. niger inoculum by turbidimetry
A suspension containing 9 × 108 spores/ mL was prepared for each assay using turbidimetry and the MacFarland scale, according to the method described by Sutton (2006), who set up that Mc Farland scale, represents specific concentrations. The spectrophotometer (Thermo Spectronic, Genesys 20) used in the present study was calibrated for the estimated microbial concentrations measuring turbidity at 540 nm (Li et al., 1993).
2.4 Culture conditions
The composition of the basal medium (BM) was NaNO3 2.0 g·L–1; KH2PO4 1.0 g·L–1; KCl 0.5 g·L–1; MgSO4 ·7H2O 0.05 g·L–1; FeCl3 0.1 g·L–1 and peptone 10 g·L–1. 500 mL of BM were autoclaved in a 1000 mL Erlenmeyer flask for 15 min at 15 psi, and the initial pH of the medium was 4.5. The medium was inoculated with 9 ×108 spores·mL–1, suspended in 0.85% sterile sodium chloride. Flasks were incubated at 30°C in a New Brunswick Scientific shaker G25 (100 rpm, 1 inch, 2.54 cm of circular orbit) for 5 days. In order to study the effect of the different carbon sources naringin, rhamnose and molasses (0.5% w·v–1) and the effect of CaCO3 and/or MgCO3 (0.01 mM) on enzyme production, both were added to the basal medium before autoclaving. The carbon sources (Bram and Solomons, 1965) and salts concentration added to the BM are shown in Table 1. All fermentations were carried out in triplicate. Weight mycelia mass, protein production and naringinase activity were determined at regular time intervals.
2.5 Growth and protein production
Growth of A. niger was determined by filtering 20 mL of each sample though Whatman No 1 filter paper, washing thoroughly with distilled water, drying overnight at 50°C and weighing its constant weight (Thammawat et al., 2008). Filter paper weigh used to filter a media flask with no fungal mycelium was used as a control. Quantification of extracellular protein was determined at regular interval times by the Bradford method (1976).
2.6 Enzyme activity
To determine the enzyme activity of the produced naringinase by A. niger a sample of 0.5 mL of each different fermentation media was mixed with 1.5mL of naringine solution (5.26mM in citrate buffer 0.05M pH 6) as substrate of the reaction. The reaction was carried out at 35°C for 15 min. Afterwards, the reaction was stopped by adding 4 mL of 3,5–dinitrosalicylic acid and boiling in a water bath for 5 min, then the samples were read at 540 nm (Miller, 1959). Every test was carried out by triplicate. A unit of enzyme (U) was defined as the amount of enzyme that releases 1μmol of glucose per minute at 35°C (pH 6.0). The naringinase activity produced by A. niger in the present work was compared with the commercial naringinase activity of Penicillium decumbens.
2.7 Protein concentration
The basal medium (BM) with the highest enzymatic activity, previously determined, was selected to concentrate protein and separated it from the culture medium. 65 g of ammonium sulfate were added for each 100 mL of medium in order to precipitate protein in a water bath, reaching a saturation of 95% according to Dawson et al. (1968). Afterwards, a dialysis again water was carrying out by using a cellulose membrane (Sigma, Chemical Co.). Eventually, the suspension was centrifuged at 19,000 × g for 5 min at 4°C; the pellet was re–suspended in citrate buffer 0.05M pH 6 and the protein content was determined by the Bradford method and the enzyme activity was followed as is described below.
2.8 Kinetic parameters
Michaelis–Menten constant (Km) values of the commercial naringinase and the naringinase produced by A. niger ATCC 1015 were evaluated by the Lineweaver–Burk, Agustinson and Wilkinson plots. Initial rates of naringin conversion (naringinase activity) were calculated by linear regression on the first data points during the first 20 min of reaction time (reducing sugars concentration versus time). The fit of the Lineweaver–Burk model (1) to experimental data was carried out using a non–linear curve–fit program in Excel for Windows, version 8.0 SR2, by minimizing the residual sum of squares between the experimental data points and the estimated values by the model. In Eq. (1), V max is the maximum reaction rate in U·mg–1, Km is the Michaelis–Menten constant in mM, V0 is the initial reaction rate in μg·min–1 and S is the substrate concentration in mM.
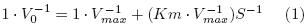
The results obtained for mycelium weight, protein content and enzymatic activity were statistically analyzed with the MINITAB13 software, by doing an analysis of variance (ANOVA) with p ≤ 0.05, using a complete general factorial design of 3x4 (carbon source and salts) with three repetitions.
3 Results and discussion
3.1 Microbial growth
The growth of Aspergillus niger ATCC 1015 was observed during seven days of fermentation for all treatments. Nevertheless, only when molasses were used as a carbon source the growth was significatively favored (p ≤ 0.05) (data not shown). Suddenly, the addition of growing factors had a positive effect on growth when molasses were used (Fig. 1). This results disagreed with Bram and Solomons (1965), who reported that the addition of growing factors like CaCO3 and MgCO3 were not relevant for Aspergillus niger NRRL 72–4 growth. Those results could be explained by the fact that they employed higher salt concentrations (0.5 and 1 %). We also observed that concentrations of 1.0 and 0.1mM did not allow any growth.

3.2 Extracelullar protein production
The production of protein excreted into the BM was dependent on the carbon sources. First of all, naringin as a carbon source favored the production of extracellular protein in each BM when was compared with molasse or rhamnose (p ≤ 0.05). Furthermore, this protein production was favored in 2.7 times by the addition of salt mix (Fig. 2). Secondly, rhamnose without salts addition exhibited the lowest protein production in the BM. Differences found between molasses and rhamnose were not significant (p > 0.05), in contrast, the opposite was observed when they were compared to naringin (Fig. 2). Moreover, when protein production in the same culture media was compared in presence of Ca2+, Mg2+ or both, the production of protein increased in every case in contrast to their respective counterpart without salts.
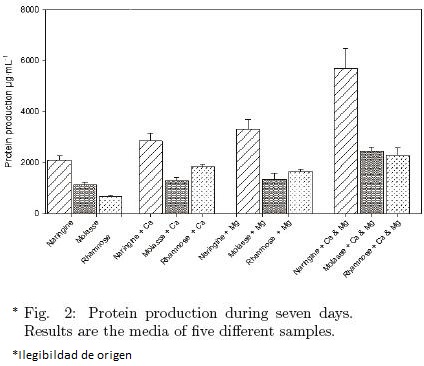
On the other hand, when are compared the BM added by naringin, no differences in the production of protein were found between no salt addition and the addition of Ca2+ or Mg2+ (p > 0.05). However, the addition of the mix of Ca2+ and Mg2+ favored the extra cellular protein production (p ≤ 0.05). In the case of molasse, a similar behavior was shown. Finally, rhamnose showed a similar behavior as well, but in addition a significative effect was observed when Ca2+ was added to the BM compared with the BM without salts. Although the difference observed when either Ca2+ or salt mix was added, no significant difference was found between them (Fig. 2). Finally, it was observed that the carbon sources had an effect on the protein production that increased in the presence of the salts mix. Furthermore, the combination of salt mix with the carbon sources had an influence in the protein production; because of that, it was found a better production of protein when the mix of salts was used with naringin than with molasse or rhamnose. It is possible that the addition of one of the salts either depends on the other (Bowen–Pope et al., 1979) or has an additive effect in the presence of some other produced enzymes. There aren't any reports about this effect in fungus but there is one related to a Mn–superoxide dismutase from rat liver and heart mitochondria reported by Pérez–Vázquez et al. (2002).
3.3 Enzymatic activity
The determination of enzymatic activity was carried out during the growth of the fungus in the BM under different conditions, by triplicate and along 5 days of fermentation, since the method quantifies the amount of glucose produced by the hydrolysis of naringinase present in the culture medium as related to a sample of pure naringin of known concentration.
Figure 3 shows the effect of the three carbon sources on enzymatic activity of naringinase. Through statistical analysis, a significant difference among them was found (p ≤ 0.05), being rhamnose without salts added, the substrate that most favored naringinase activity. Furthermore, no significant differences were observed between the samples with Mg2+ or the mix of salts (p > 0.05) in any of the different carbon sources utilized. In contrast, when Ca2+ was added to the BM significant differences were found when naringin and molasses were employed as a carbon sources. However, the enzymatic activity of BM with naringin and Ca2+ was a slightly less strong than the enzymatic activity of BM added with rhamnose without salts (Fig. 3). On the other hand, when the effects of the salts were analyzed in each carbon source, we did not found any significant difference about enzymatic activity in the BM with naringin with or without salts. Moreover, a similar behavior was shown by molasse and rhamnose, when were employed as a carbon sources.
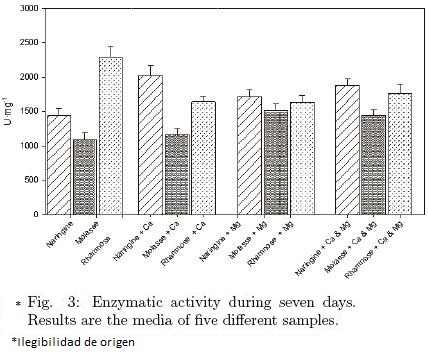
The maximum activity found by rhamnose with no added salts may be explained by the report of Puri et al. (2005), who stated that, when rhamnose was added to the culture media, A. niger produced high amounts of naringinase. This disagrees with the report by Elinbaum et al. (2002), who mention that naringinase is a better inductor than rhamnose in the production of naringin using A. terreus in solid fermentation. Furthermore, they suggest that solid fermentation gives better results in the production of naringinase than its liquid counterpart as reported too by Viniegra et al. (2003) where shown effective growth of fungi and production of enzymes in solid–state fermentation (SSF) in comparison to liquid cultivation.
The enzymatic production obtained in the present work may be explained by the economic theory of microorganisms (Koch, 1985), which establishes that the production of an induced enzyme is only favored when it allows the microorganism to obtain more nutrients for its growth and multiplication. According to Allison and Vitousek (2005), extracellular enzymes such as naringinase are the principal means by which microorganisms degrade complex organic compounds into smaller molecules that may be assimilated. In this experiment, it was observed that rhamnose is a complex compound that favors the production of enzymes and that molasses, by being easily assimilated, induces enzymatic activity only slightly.
It was found that the production of protein along fermentation time was not proportional to the enzymatic activity (Fig. 2 and Fig. 3), since a higher protein concentration did not produce a higher enzymatic activity.
3.4 Enzymatic activity of the selected culture broth
Figure 3 shows the statistical analysis of the different carbon sources effect on the enzymatic activity. The highest activity was founded with rhamnose as a carbon source and without any salt added. To determine the kinetic constants the protein excreted after 24 hrs to the BM added with rhamnose was collected and concentrated. The kinetic characteristics found were Vmax = 13.24 ± 0.28 U·mg–1 and Km = 1.6 ± 0.015 mM. On one hand, despite the low activity founded (0.009 U·mg–1) was better than other reported by Puri et al (2005), who found a naringinase activity of 3.99 × 10–4 U by A. niger MTCC 1344 in a BM added with rhamnose. Elinbaum et al. (2002), reported a better enzyme activity by the α–L–ramnosidasa from A. terreus (1.7 U·mL–1), however in this case there are many differences in comparison with the methodology employed in the present work. First of all the BM was added with sugar cane bagasse as a carbon source. Secondly the activity was tested in different conditions (succinic acid/sodium succinate buffer 50mM, pH= 5 at 30°C) and finally the unit was defined as the amount of the enzyme that catalyst the release of 1 μmol of p–nitrophenol·min–1, which implies a different methodology for the determination of the enzyme activity, regarding to the one employed in the present work. Bram and Solomons (1965) reported a naringinase activity of 94U·mL–1 by A. niger NRRL 72–4 in a BM added with 2% corn steep liquor and 2.5% yeast extract.
Regarding the kinetic characteristics, many authors have reported higher values of Vmax than the reported in the present work (Table 2). Gallego et al. (2001) reported 91U·mg–1 for the activity of the α–L–rhamnosidasa from A. terreus and the kinetic constants were Vmax= 84U·mg–1 and Km=0.17mM for the purified enzyme. Our results shown a low Vmax and higher Km for the raw enzyme. Moreover,  ekero
ekero lu et al (2006) reported a Vmax = 0.45 μmol·mg–1 min–1 which is lower than the obtained in the present work, and a Km= 1.22 mM, which is slightly lower than the one observed in the present study, meaning that their naringinase has a litter higher substrate affinity. Finally Manzanares et al (2001) reported an activity for a commercial preparation of the α–L–rhamnosidasa from A. niger of Vmax=20.6U·mg–1 and a Km=2.9U·mg–1, which means that the activity obtained in the present work has a better affinity than the obtained by Manzanares et al. (2001).
lu et al (2006) reported a Vmax = 0.45 μmol·mg–1 min–1 which is lower than the obtained in the present work, and a Km= 1.22 mM, which is slightly lower than the one observed in the present study, meaning that their naringinase has a litter higher substrate affinity. Finally Manzanares et al (2001) reported an activity for a commercial preparation of the α–L–rhamnosidasa from A. niger of Vmax=20.6U·mg–1 and a Km=2.9U·mg–1, which means that the activity obtained in the present work has a better affinity than the obtained by Manzanares et al. (2001).

In the present study, the values of Vmax and Km for the naringinase produced by A. niger ATCC1015 match the ones found for P. decumbens naringinase (blank), meaning that in spite of having a low protein production, the constants were of the same order (Table 2). Furthermore, it was observed that the conditions that favor the growth of A. niger, the production of excreted protein, and the enzymatic activity are different.
Conclusions
Under the conditions of the present work the growth and protein production were not the optimal to promote the highest enzymatic activity of the resulting naringinase, since the best growth was observed when using molasses and CaCO3, while the best protein production was obtained with naringin and a mix of the salts. The higher enzymatic activity was found when using rhamnose as carbon sources with no salt addition. The naringinase activity founded under the conditions of this study shows similar kinetic characteristics regarding to commercial naringinase.
Acknowledgements
The authors express their gratitude to Ingenio San Miguelito Veracruz for the sample of molasse. This work was supported by grant from Instituto Politécnico Nacional.
Referencias
Allison, S.D., Vitousek, P.M. (2005). Response of extracellular enzymes to simple and complex nutrient inputs. Soil Biology and Biochemistry 37, 937–944. [ Links ]
Birgisson, H., Hreggvidsson, G.O., Fridjónsson, O.H., Mort, A., Kristjánsson, J.K., Mattiasson, B. (2004). Two new thermostable α–L–rhamnosidases from a novel thermophilic bacterium. Enzyme and Microbial Technology 34, 561–571. [ Links ]
Bowen–Pope, D.F., Vidair, C., Sanui, H., Rubin, A.H. (1979). Separate roles for calcium and magnesium in their synergistic effect on uridine uptake by cultured cells: Significance for growth control. Cell Biology 76 (3),1308–1312. [ Links ]
Bradford, M.M. (1976). Soluble protein determination. Analytical Biochemistry 72, 248–254. [ Links ]
Bram, B., Solomons, G.L. (1965). Production of the enzyme naringinase by Aspergillus niger. Applied Microbiology 13, 842–845. [ Links ]
Chang, H.Y., Lee, Y.B. Bae, H.A., Huha, J.Y., Nama, S.H., Sohnb, H.S., Leec, H.J., Leea, S.B. (2011). Purification and characterisation of Aspergillus sojae naringinase: The production of prunin exhibiting markedly enhanced solubility with in vitro inhibition of HMG–CoA reductase. Food Chemistry 124, 234–241. [ Links ]
Dawson, R.M.C., Elliott, D.C., Elliott, W.H., Jones, K.M. (1968). Data for Biochemical Research. (2nd ed.). Oxford University Press, London. [ Links ]
Del Nobile, M.A., Piergiovanni, L., Buonocore, G.G., Fava, P., Puglisi, M.L., Nicolais, L. (2003). Naringinase immobilization in polymeric films intended for food packaging applications. Journal of Food Science 68, 2046–2049. [ Links ]
Elinbaum, S., Ferreyra, H., Ellenrieder, G., Cuevas, C. (2002). Production of Aspergil lus terreus α–L–rhamnosidase by solid state fermentation. Letters in Applied Microbiology 34, 67–71. [ Links ]
Gallego, M.V., Piñaga, F., Ramón, D., Vallés, S. (2001). Purification and characterization of an α–L–rhamnosidase from Aspergillus terreus of interest in winemaking. Journal of Food Science 66, 204–209. [ Links ]
Kishi, K. (1955). Production of naringinase from Aspergillus niger. Kagaku to Kogyo (Chemistry and Industry, Japan) 29, 140. [ Links ]
Koch, A.L. (1985). The macroeconomics of bacterial growth. In: Bacteria in their Natural Environments, (Fletchjer M and G.D. Floodgate, eds.), Pp. 1–42. Academic Press, London. [ Links ]
Li, R.C., Nix, D.E., Schentag, J.J. (1993). New turbidimetric assay for quantification of viable bacteria densities. Antimicrobial Agents and Chemotherapy 2, 371–374. [ Links ]
Manzanares, P., Van den Broeck, H.C., de Graaff, L.H., Viseer, J. (2001). Purification and characterization of two different α–L–rhamnosidases, RhaA and RhaB, from Aspergillus aculeatus. Applied Environmental and Microbiololgy 67, 2230–2234. [ Links ]
Miller, G.L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31, 426–428. [ Links ]
Norouzian, D., Hosseinzadeh, A., Nouri Inanlou, D., Moazami, N. (2000). Production and partial Purification of naringinase by Penicillium decumbens PTCC 5248. World Journal of Microbiology and Biotechnology 16, 471–473. [ Links ]
Pérez–Vázquez, V., Ramírez, J., Aguilera–Aguirre, L., González–Hernández, J. C., Clemente–Guerrero M., Manzo–Avalos, S.,Uribe, S., Saavedra–Molina, A. (2002). Effect of Ca+2 and Mg+2 on the Mn–superoxide dismutase from rat liver and heart mitochondria. Amino Acids 22, 405–416. [ Links ]
Puri, M., Banerjee, A., Banerjee, U.C. (2005). Optimization of process parameters for the production of naringinase by Aspergillus niger MTCC 1344. Process Biochemistry 40, 195–201. [ Links ]
Puri, M. and Kalra, S. (2005). Purification and characterization of naringinase from a newly isolated strain of Aspergillus niger 1344 for the transformation of flavonoids. World Journal of Microbiology and Biotechnology 21, 753–758. [ Links ]
 ekero
ekero lu, G., Fadilo
lu, G., Fadilo lu, S., Gö
lu, S., Gö ü
ü , F. (2006). Immobilization and characterization of naringinase for the hydrolysis of naringin. European Food Research and Technology 224, 55–60. [ Links ]
, F. (2006). Immobilization and characterization of naringinase for the hydrolysis of naringin. European Food Research and Technology 224, 55–60. [ Links ]
Sutton, S. (2006). Measurement of Cell Concentration in Suspension by Optical Density. Pharmaceutical Microbiology Forum Newsletter 12, 9–13. [ Links ]
Thammawat, K., Pongtanya, P., Juntharasri, V., Wongvithoonyaporn, P. (2008). Isolation, preliminary enzyme characterization and optimization of culture parameters for production of naringinase isolated from Aspergillus niger BCC 25166. Journal of Natural Science 42, 61–72. [ Links ]
Vila Real, H.J., Alfaia, A.J., Calado, A.R.T., Riberiro, M.H.L. (2006). High pressure–temperature effects on enzymatic activity: Naringin bioconversion. Food Chemistry 102, 565–570. [ Links ]
Viniegra G., Favela E., Aguilar C.N., Romero–Gómez S.J., Diaz–Godínez G., Augur C. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering Journal 13, 157–167 [ Links ]
Zverlov, V.V., Hertel, C., Bronnenmeir, K., Hroch, A., Kellermann, J., Schwarz, W.H. (2000). The thermostable α–L–rhamnosidase RamA of Clostridium stercorarium: biochemical characterization and primary structure of a bacterial α–L–rhamnoside hydrolase, a new type of inverting glycoside hydrolase. Molecular Microbiology 35, 173–179. [ Links ]














