Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.9 no.1 Ciudad de México abr. 2010
Materiales
Photothermal selective coatings of black molybdenum
Recubrimientos selectivos fototérmicos de molibdeno negro
F. González, E. Barrera C.* and R. Rosas C.
Departamento de lngeniería de Procesos e Hidráulica, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco No. 186, Col. Vicentina, Iztapalapa, C.P. 09340, México, D.F. México. *Corresponding author. E-mail: ebc@xanum.uam.mx Tel. 58044644-107 Fax 58044900.
Received 10 of July 2009;
Accepted 29 of January 2010
Abstract
Thin and adherent coatings of molybdenum black solar selective coatings have been produced on copper substrates by immersion in solutions with different concentration of ammonium molybdate. The copper sheet was polished prior to immersion in the molybdate solution. XRD analyses showed that the coating consists of different oxides of copper and molybdenum. The maximum solar absorptance of the resulting molybdenum black coating was 0.87 for which the thermal emittance was 0.45. The best selectivity of 4.05 was obtained for a coating made in three days in an 8.0 mM solution of ammonium molybdate, for which the absorptance was 0.81 and thermal emittance, 0.2.
Keywords: solar selective coatings, optical properties, microstructural properties, chemical bath, molybdenum black.
Resumen
Se produjeron recubrimientos solares selectivos delgados de molibdeno negro sobre sustratos de cobre. La técnica de crecimiento empleada fue por inmersión en soluciones con diferente concentración de molibdato de amonio. Los sustratos de cobre fueron pulidos antes de introducirse en la solución con el fin de garantizar la adherencia. El análisis de difracción de rayos X revelo que los recubrimientos consisten de óxidos de cobre y molibdeno. La máxima absortancia solar de los recubrimientos de molibdeno negro resultó ser de 0.87 con un valor correspondiente de 0.45 para la emitancia térmica. La mejor selectividad alcanzada fue de 4.05 para el recubrimiento depositado durante tres días en una solución 8.0 mM de molibdato de amonio, en el que la absortancia fue de 0.81 y la emitancia térmica de 0.2.
Palabras clave: recubrimientos solares selectivos, propiedades ópticas, propiedades microestructurales, baño químico, molibdeno negro.
1. Introduction
Selective coatings formation in a simple and reproducible way is a key issue for photo thermal conversion of solar energy. These coatings are the technological base to produce solar collectors and other interestingly applications (Dali and Keil 1975). A spectrally selective surface should capture the maximum solar energy and should have minimum emittance for thermal IR radiation. To enhance the total solar absorptance of the coating, the high intensity visible spectral region should have the lowest possible reflectance and, to suppress the emittance, the thermal radiation should have highest possible reflectance.
The photo thermal materials of flat solar collectors regularly are made of copper or aluminum. However, these metals need to be coated with tight bonded selective films. There are a lot of methods to prepare the coatings, and many types of these ones.
Molybdenum black coatings have been prepared by different synthesis techniques, including electrochemical deposited coatings (Anicai et al. 2008, Patil et al. 2008) and chemical conversion method (Jahan and Smith 1997, Jahan and Smith 1992). The substrates used in these reports were aluminum foils, zinc sheets and nickel cobalt-plated copper sheets. The typical optical values for solar absortance and thermal emittance have values between 0.84 and 0.93 and between 0.13 and 0.25, respectively.
In this work we present a straightforward method to prepare black molybdenum by immersion in a chemical solution bath. We establish the optimum condition to grow the selective coatings on copper sheets. Additionally optical and chemical characterizations were done.
2. Experimental
2.1. Coating preparation
Commercially available copper sheets of thickness around 1 mm were cut in pieces of around 10 cm2 and used as substrates. In order to obtain uniform coatings, the copper surface was polished by using SiC paper of grade 1200. Then the substrates were washed with distilled water and dried with gaseous N2. Following the cleaning step, the copper sheets were immersed into the chemical bath to grow the coating at room temperature. The solution consisted of ammonium paramolyb-date (NH4)6Mo7O24·4H2O and deionized water. The initial pH solution (before copper sheets immersion) was 4.5. Looking for the optimum result (highest value of the ratio α/ε) different concentration of (NH4)6Mo7O24·4H2O in the solution, bath A (8.0 mM), bath AB (8.0 mM), bath B (4.0 mM) and bath CB (2.0 mM) and deposition time, (1 to 4 days) were used. After deposition of the coatings, they were washed with distilled water and dried with gaseous N2. The color of the coating depended on the time of immersion. As this time increased, the coating became darker. All coatings were grown at room temperature. Concerning the uniformity of the coatings, due to the nature of the chemical deposition we have obtained, to the naked eye, uniform deposition over the whole area where the solution and substrate are in contact. Typical coating rate is 1 μm/day.
2.2. Characterization
The composition of the coatings was determined by X-ray powder diffraction at room temperature with CuK α radiation by using a D 500 Siemens diffractometer. The solar absorption (αs) and the emittance of the coatings were determined by using the Duffie and Beckman approach (Duffie and Beckman 1991). The total hemispherical reflectance, between 0.3 and 2.5 μm corresponding to the solar spectrum was recorded by using a Cary 5 E spectrophotometer equipped with an integrating sphere operating in the reflectance mode. The emittance was obtained from specular reflectance measurements in the infrared region (2.5-25 μm) using a Nicolet 750 FTIR spectrometer and, a gold mirror was employed as the 100 % reflectance reference. Chemical composition of coatings was determined by SEM-EDS using a JEOL 5900 LV microscope with OXFORD ISIS equipment. An Ambios atomic force microscope in non-contact mode was used to investigate the surface morphology of the coatings. The RMS roughness was calculated from the experimental data.
3. Results and discussion
3.1. Structure and compositional analysis
The structure and composition are of primary importance to understand the selective properties of a coating. The XRD patterns of the deposited Mo-black coatings indicate their polycrystalline and multi compound nature (Fig. 1a). From the Bragg reflections it was possible to determine the presence of (NH4)2Cu(MoO4)2 (JCPDS 40-1490), Cu3Mo2O9 (JCPDS 22-0609) and MoO3 (JCPDS 65-7675). Actually, from a literature survey we found that the entire identification of the compounds in other Mo-black coating is not trivial, but a complicated work (Jahan et al. 1997). In the previous reports it was found that the Mo-black coatings were both, non-crystalline (Potdar et al. 1987) and polycrystalline (Jahan et al. 1997) in nature. However all of them agree with the fact that Mo has a high oxidation state (between 5 and 6+); for example Potdar et al. using XPS reported a non-crystalline phase, which is compatible with MoO3, and Jahan et al., found evidence of the Mo4O11 crystalline phase in the as prepared coatings from electron diffraction. The effect on the optical properties of the different compounds present in the coatings is an open question, and further work must be done to establish it. The compounds with copper provide evidence of a probable activation of this element in the deposition process, as is expected in the chemical conversion process. The EDS analysis performed on samples for different deposition times, reveals the presence of the expected elements, i.e., O, Mo and Cu (see Fig.1b).

3.2. Morphology
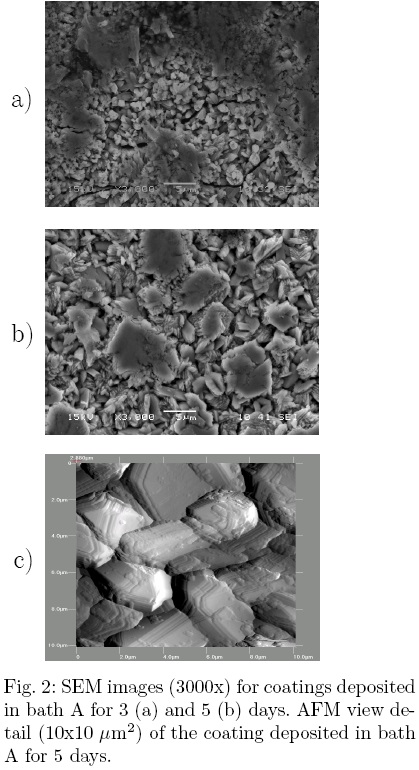
The surface morphology was studied by scanning electron microscopy (SEM) and atomic force microscopy (AFM). A typical surface morphology images are shown in Fig. 2. The surface consists of a large number of flake-like grains, ranging in size from 0.1 to 4 μm. From figs. 2 (a) and (b) it is observed that as the deposition time increases, the grain size increases too. These features: shape and size can be explained by the mechanism of grain growth. In Fig. 2 (c) is shown an AFM image of 10 x 10 μm2 area of the coating. The grains exhibit a layer-by-layer grain growth; in that way it is possible to increase the size preserving the shape at anytime through the deposition process.
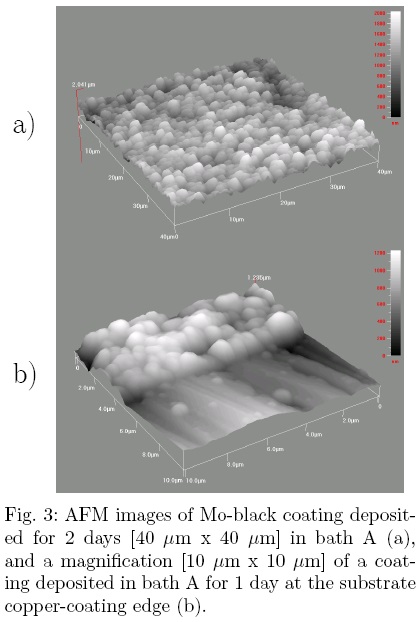
In Fig. 3, AFM images deposited in bath A for 1 day are depicted. The RMS roughness of the Fig. 3(a) has a value of 286 nm. As the surface roughness is of the order of solar radiation wavelengths, it does not affect the emittance value significantly. The rate of coating deposition was estimated from Fig. 3(b), resulting in 1 μm per day.
3.3. Optical properties
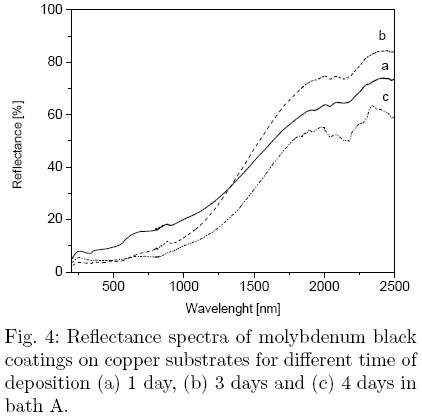
The total reflectance spectra of the Mo-black coatings on copper substrates are shown in Fig. 4 for 1, 3 and 4 days of deposition time in bath A. No peaks are observed in the region below 0.7 μm, but a monotonous increase in the reflectance is present for all spectra. The coatings have lowest reflectance in the UV and visible region. As the deposition time increases, the reflectance has lower values in that region, due to the absorption improvement; however, in the near infrared (NIR) region, the reflectance reaches its maximum value for deposition during 3 days, and then decreases as the deposition time increases. This effect is related with the thickness of the coating. The thinner coatings appear to be transparent to IR radiation and the thermal radiation is reflected from the substrate itself. As the coating thickness increases, the coating becomes opaque to IR radiation and the emittance increases. The values of the absortance and the emittance for different deposition time are presented in Table 1. The deposition time was found to be the critical parameter to achieve minimum emittance with the best selectivity factor, 4.05. The absorption increases gradually and reaches a maximum value of 0.87, while the emittance has a minimum value of 0.2 reached at different durations, depending on the bath composition. The values for the solar absortance and the thermal emittance in the coating with improved selectivity are comparable with those reported in the literature for similar coatings.
However, the distinguishable feature of the method reported in the present work is its simplicity to grow the selective coatings. Finally, we can say that our coatings exhibit good adhesion to the substrate and preliminary results concerning thermal stability have shown no appreciable changes in optical properties for heat treated coatings up to 150 ° C. Manufacturing selective coatings through our process is non expensive and easy, compared with other techniques such as sputtering or electro-deposition. Despite the consuming time to achieve an optimal coating, the chemical conversion does not require additional resources, thus complying with the processes demanded in the industry.
Conclusions
Deep black coatings consisting of a mixture of molybdenum compounds, MoO3, Cu3Mo2O9 and (NH4)2 Cu(MoO4)2, were obtained by using a chemical oxidation dipping technique from ammonium molybdate diluted solutions. Samples with 1, 2, 3, 4 dipping days were made having interesting solar optical properties and, for the simple way to synthesize the material it is a very attractive technique to made coatings in a large surface areas for several solar applications.
The rate of deposition of molybdenum oxide black films on copper substrate is higher in the bath A in relation to bath B and C. Bath A gives a deposition rate of around 1 micron in the molybdenum black coating per day. Currently experiments are being conducted in our laboratory in order to get a better understanding on the effect of molybdenum concentration, bath temperature and deposition times.
Acknowledgement
This work was partially carried out with the financial support of CONACYT, project 60781-Y.
References
Anicai L. Pertache A. and Visanb T. (2008) Thin black layers on aluminum substrate - electrochemical synthesis and characterization. Surface and Interface Analysis 40, 818-821. [ Links ]
Dali C.M. and Keil R.G. (1975). On the anodic oxidation of molybdenum. Journal of The Electrochemical Society 122, 350-353. [ Links ]
Duffie J.A. and Beckman W. A. (1991). Solar engineering of thermal processes. John Wiley and Sons, New York. [ Links ]
Jahan F. I. and Smith B.E. (1997). Characterization of molybdenum black coatings on zinc substrates. Journal of Materials Science 32, 3869-3874. [ Links ]
Jahan F. I. and Smith B.E. (1992). Investigation of solar selective and microstructural properties of molybdenum black immersion coatings on cobalt substrates. Journal of Materials Science 27, 625-636. [ Links ]
Patil R.S., Uplane M.D. and Patil P.S. (2008). Electrosynthesis of electrochromic molybdenum oxide thin films with rod-like features. International Journal of Electrochemical Science 3, 259-265. [ Links ]
Potdar H.S., Mandale A. B., Sathaye S.D. and Sinha A.P.B. (1987). Characterization of solar selective molybdenum black films. Journal of Materials Science 22, 2023-2031. [ Links ]














